<<
Back to Kanarev's Physchemistry Book Index
13. COLD FUSION BY PLASMA ELECTROLYSIS OF WATER
Cold nuclear fusion is the first hypothesis of a source of
additional energy in heavy water electrolysis. Fleischmann and Pons, the
American electrochemists, are the authors of this hypothesis . They reported
about it in 1989 [50], [67], [83]. Since that time a large number of experiments has
been carried out in order to obtain additional energy from water [73], [83]. The Japanese
investigators proved by experiment availability of the cold nuclear fusion
process during plasma electrolysis of water
[51].
The results of these experiments are discussed at the international conferences
devoted to cold nuclear fusion, that’s why there is every reason to analyse
this hypothesis [187].
In order to check this hypothesis, the following experiments were performed.
Two cathodes were made of iron with mass of 18.10 g and 18.15 g. The first
cathode operated during 10 hours in KOH solution; the second cathode operated
during the same period in NaOH solution. Mass of the first cathode remained
unchanged, mass of the second one was reduced by 0.02 g.
Tadahiko Mizuno,
the famous Japanese scientists (the co-author of this article), who works at
the Division of Quantum Energy Engineering Research group of Nuclear System
Engineering, laboratory of Nuclear Material System, Faculty of Engineering,
Hokkaido University, Kita-ku, North 13, West-8 Sapporo 060-8628, Japan, kindly
agreed to perform chemical analysis of the cathode samples with the help of the
nuclear spectroscopy method (EDX). Here are the results of his analysis. The
content of chemical elements on the surface of non-operating cathode is as
follows (Table 46).
Table 46. Chemical
composition of the cathode surface prior its operation in the solution
|
Element |
Fe |
|
% |
99.90 |
The new chemical
elements have appeared on the working surface of the cathode, which works in
KOH solution (Table 47).
Table 47. Chemical
composition of the surface of the cathode, which operates in KOH solution
|
Element |
Si |
K |
Cr |
Fe |
Cu |
|
% |
0.94 |
4.50 |
1.90 |
93.00 |
0.45 |
The chemical
composition of the surface of the cathode, which operates in NaOH. Has proved
to be different (Table 48).
Table 48. Chemical composition of the surface
of the cathode, which operates in NaOH solution
|
Element |
Al |
Si |
Cl |
K |
Ca |
Cr |
Fe |
Cu |
|
% |
1.10 |
0.55 |
0.20 |
0.60 |
0.40 |
1.60 |
94.0 |
0.65 |
Numerous
experiments show that up to 50% of additional thermal energy are generated
during the plasma electrolysis of water, it is less than the results of the
calculations originating from the existing cold nucleus theories. That’s why it is necessary to
analyse energetics of the particle creation process during the atomic nucleus
transmutation.
Having considered the
model of the electron we have found out that it can exist in a free state only
when it has a definite electromagnetic mass. Being combined with the atomic
nucleus it emits a part of energy in the form of the photons, and its
electromagnetic mass is reduced. But stability of its condition does not become
worse, because the energy carried away by the photons is compensated by binding
energy of the electron in the atomic nucleus.
If the ambient
temperature is increased, the electron begins to absorb the thermal photons and
to pass to higher energy levels of the atom reducing binding with it. When the
electron becomes free, it interacts with the atom only if the ambient
temperature is reduced. As this temperature is reduced, it will emit the
photons and sink to lower energy levels.
If the electron is
in a free state due to an accidental external influence on the atom and the
environment has no photons, which are necessary for it to restore its mass, it
begins to absorb the ether from the environment and to restore its constants in
such a way: mass, charge, magnetic moment, spin and radius of rotation. The
electron acquires the stable free state only after it has restored its all
constants.
Thus, if an
interchange of the free state and binding state with the atom takes place due
to the accidental influences on the atom, the electron restores its
electromagnetic mass every time due to absorbing the ether. It means that
actually it plays the role of a converter of the ether energy into the thermal
photon energy.
The Japanese
investigators Ohmori and Mizuno [51] registered neutron radiation during plasma
electrolysis of water and reported that not only the nuclear process, but the
process of the electron capture by the free protons can be the source of this
radiation.
As hydrogen plasma
is generated during the plasma electrolytic process of water electrolysis,
there exists a tendency of the capture of the free electrons by them.
It is known that
rest mass of the electron is ![]() , rest mass of the proton is
, rest mass of the proton is ![]() , and rest mass of the neutron is
, and rest mass of the neutron is ![]() . The difference between the mass of the neutron and the mass of the
proton is equal to
. The difference between the mass of the neutron and the mass of the
proton is equal to ![]() . It is
. It is ![]() of the mass of the electron.
Thus, the proton should capture 2.531 electrons in order to become the neutron.
The question arises at once: what will happen to the remained of electron mass
of the mass of the electron.
Thus, the proton should capture 2.531 electrons in order to become the neutron.
The question arises at once: what will happen to the remained of electron mass ![]() ? The disturbed balance of masses in this process is explained by modern
physics in a simple way: a neutrino is created.
? The disturbed balance of masses in this process is explained by modern
physics in a simple way: a neutrino is created.
As the neutrino has
no charge, it is very difficult to register it. If the neutrino takes the
excess mass away or replenish the lacking one, can the elementary particles
execute this process by themselves?
As the photons are
emitted and absorbed only by the electrons, the proton, which absorbs the
electrons, cannot convert the remainder of mass of the third electron into the
photon. If the electron is absorbed by the third one and gives more than a half
of its mass to the proton in order to convert it into the neutron, the
remaining part of mass ![]() of the electron,
which has no possibility to become the photon, is converted into a portion of
the ether, which “is dissolved” and mixed with the ether in the space. The fact
that plasma has no photons with the mass corresponding to the part of mass of
the third electron, which has not been absorbed by the proton during its
conversion into the neutron, can serve as a proof of such affirmation. Let us
calculate energy of such photon.
of the electron,
which has no possibility to become the photon, is converted into a portion of
the ether, which “is dissolved” and mixed with the ether in the space. The fact
that plasma has no photons with the mass corresponding to the part of mass of
the third electron, which has not been absorbed by the proton during its
conversion into the neutron, can serve as a proof of such affirmation. Let us
calculate energy of such photon.
The difference the
mass of the neutron and the proton is equal to ![]() . If we subtract this value from the mass of three electrons, we’ll get
mass
. If we subtract this value from the mass of three electrons, we’ll get
mass ![]() , from which the photon should be formed
, from which the photon should be formed
![]() (308)
(308)
If the photon is
formed from this remainder of mass ![]() , its energy will be:
, its energy will be:
![]() (309)
(309)
This value of
energy corresponds to roentgen spectrum, that’s why the creation of each free
neutron should be accompanied by the creation of one roentgen photon. If it
does not take place, we have two opportunities: the first one – we should think
that in the case when the neutron is created, the neutrino was formed from mass
![]() and flew away in the unknown
direction; the second one – there were no conditions for the formation of the
photons in the process being considered, and mass
and flew away in the unknown
direction; the second one – there were no conditions for the formation of the
photons in the process being considered, and mass ![]() , which failed to be formed as a particle, “was dissolved” in
the ether. Which variant is closer to the truth? There is no exact answer, but it is known that the Japanese
scientists registered only neutron radiation with intensity of 50,000 neutrons per
second, and they failed to register roentgen radiation [51].
, which failed to be formed as a particle, “was dissolved” in
the ether. Which variant is closer to the truth? There is no exact answer, but it is known that the Japanese
scientists registered only neutron radiation with intensity of 50,000 neutrons per
second, and they failed to register roentgen radiation [51].
If in this process
the roentgen photons were created, they would not exceed heat efficacy of the
plasma electrolytic process, because they would not be the thermal photons. The
thermal photons are radiated and absorbed when the electrons make the energy
transitions to the energy levels, which are the most remote from the atomic
nuclei, where the infrared photons and neighbouring ones from the optical range
of the spectrum with energies of ![]() (0.001-3.3) eV are generated (Table 34).
(0.001-3.3) eV are generated (Table 34).
Thus, the neutron
fusion processes during the plasma water electrolysis will not generate
additional thermal energy. But the appearance of the neutrons in plasma will
promote the formation of the deuterium nuclei (Fig. 21, b) and, possibly, of
tritium (Fig. 21, c). As during these processes the mass balance is almost
not changed, we have no reason to
anticipate the appearance of additional energies during the formation of deuterium
and tritium.
In order to become
a proton, the neutron should emit something with mass ![]() =23.058×10-31
kg. Let us concert this mass into energy.
=23.058×10-31
kg. Let us concert this mass into energy.
![]() (310)
(310)
This energy
corresponds to the photons of the gamma range. Thus, if during plasma water
electrolysis the process of helium atom formation takes place, it should be
accompanied by gamma radiation. If there is no such radiation, and the helium
atoms are still formed, the above mentioned portion of mass ![]() is taken away by neutrino
or this mass, which has no possibility to become a photon, “is dissolved” in
the environment, i.e. it passes into a state of ether [84]. As the roentgen
photons and the gamma photons are not the thermal ones, this process does not
give excessive thermal energy.
is taken away by neutrino
or this mass, which has no possibility to become a photon, “is dissolved” in
the environment, i.e. it passes into a state of ether [84]. As the roentgen
photons and the gamma photons are not the thermal ones, this process does not
give excessive thermal energy.
Let us carry out the
preliminary analysis of the data being obtained (Tables 46, 47, 48).
As iron is the
cathode material, the nuclei of its atoms are the targets of the atomic nuclei
of potassium, alkaline metal. During the transmutation of the iron nuclei (Fig.
96, b), the atomic nuclei of chromium (Fig. 96 a) and the
atomic nuclei of copper (Fig. 96, c) are formed.
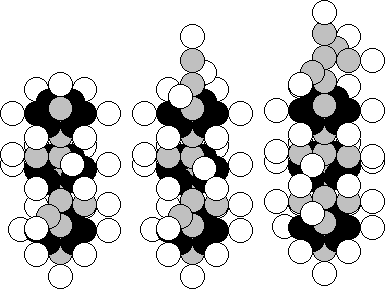
a) Cr (24,28) b) Fe (26,28) c) Cu (29,34)
Fig. 96. Diagrams of the atomic nuclei of: a) chromium, b) iron, c)
copper
When the atomic
nucleus of iron (Fig. 96, b) pass into the atomic nucleus of chromium
(Fig. 96, a), two protons and two neutrons are released; two atoms of deuterium
or one atom of helium can be formed from them. If the neutrons pass into the
protons, four atoms of hydrogen are formed.
It is easy to see
(Fig. 96) that the atomic nucleus of iron (Fig. 96, b)
should lose two upper protons and two neutrons in order to pass into the atomic
nucleus of chromium (Fig. 96, a).
Three additional
protons and six neutrons (total 9 nucleons) are required for the formation of
the atomic nucleus of copper (Fig. 96, c) from the atomic nucleus of iron. As there
are chromium atoms, which, as we think, are formed from the atomic nuclei of
iron, on the cathode surface (Table 47) 4fold more than the atoms of copper,
the solution is sure to have superfluous protons and neutrons of the destroyed
atomic nuclei of iron, and we can determined their approximate relative
quantity.
Let us suppose that
four nuclei of the iron atoms pass into the nuclei of the chromium atom. The
total quantity of free protons and neutrons (nucleons) is equal to 16. As one
atom of copper falls on each four atoms of chromium, 9 nucleons are spent for
the formation of one nucleus of the copper atom, and 7 nucleons remain free.
Let us see what is
formed when the nucleus of the potassium atom is destroyed. Potassium is
situated in the first group of the fourth period of the periodic law. Its
nucleus contains 19 protons and 20 neutrons (Fig. 97, a).
In Fig. 97, a, we
can see a weak link of the nucleus of the potassium atom. It is situated in the
middle of its axis neutrons. When the transmutation of the nuclei of the
potassium atoms takes place, the nuclei of the oxygen atoms can be formed (Fig.
97, b) as well as its isotopes and the nuclei of the silicon atoms (Fig. 97, c).
The analysis of the
structure of the nuclei of the potassium atom (Fig. 97, a) shows
that its is the most probable source of the nucleus of the silicon atom (Fig. 97, b),
which atoms appear on the cathode (Table 47).
It is easy to count
that during the destruction of one nucleus of the potassium atom and the
creation of one nucleus of the silicon atom 5 free protons and 6 free neutrons,
i.e. 11 nucleons, are formed.
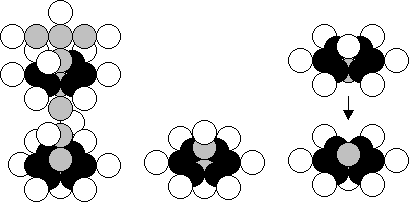
a) K (19,20) b) O (8,8) c) Si (14,14)
Fig. 97. Diagrams of the atomic nuclei of: a)
potassium, b) oxygen, c) silicon
Thus, the transmutation of the nuclei of the
iron atoms and the potassium atoms results in the formation of free protons and
neutrons. As the protons cannot exist in free state, the hydrogen atoms are
created from them. If the protons are connected with the neutrons after the
destruction of the nuclei of the iron atoms and the potassium atoms, the
formation of deuterium, tritium and helium is possible.
In any of these
cases, the atoms and the molecules of hydrogen are formed. We have already
shown that the processes of fusion of the atoms and the molecules of hydrogen
and its isotopes result in occurrence
of additional thermal energy.
 Let us pay attention
to the main fact – absence of the sodium atoms in the cathode material. It is
natural that the potassium atoms have appeared on the cathode, which operated
in KOH solution (Table 48). Why are no sodium atoms on the cathode, which
operated in NaOH solution? The answer is as follows: the nuclei of the sodium
toms are completely destroyed during the plasma electrolytic process. The
presence of potassium on the surface of the cathode, which operated in NaOH
solution (Table 48), can be explained by insufficient ablution of the reactor
after the operation with KOH solution.
Let us pay attention
to the main fact – absence of the sodium atoms in the cathode material. It is
natural that the potassium atoms have appeared on the cathode, which operated
in KOH solution (Table 48). Why are no sodium atoms on the cathode, which
operated in NaOH solution? The answer is as follows: the nuclei of the sodium
toms are completely destroyed during the plasma electrolytic process. The
presence of potassium on the surface of the cathode, which operated in NaOH
solution (Table 48), can be explained by insufficient ablution of the reactor
after the operation with KOH solution.
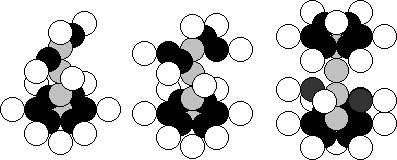
a) Na (11,12) b) Al (13,14) c) Cl (17,18)
Fig. 98. Diagrams of the
atomic nuclei of: a) sodium, b) aluminium, c) chlorine
As free protons and
neutrons appear during the destruction of the nucleus of the sodium atom, some
nuclei of this element begin to form the atomic nuclei of aluminium (Fig. 98, b), chlorine
(Fig. 98, c) and calcium
(Fig. 99).
But not all free
protons and neutrons are spent for the construction of the atomic nuclei of
aluminium, chlorine and calcium. A part of them is spent for the hydrogen atom
formation.
If we knew the
total quantity of transmutating atomic nuclei of iron, potassium and sodium as
well as the exact composition of the gases generated during the plasma electrolytic
process, it would be possible to determine the atomic nuclei being formed from
additional nucleons. Now we can only suppose that the majority of new nuclei
are the protons, i.e. the nuclei of the hydrogen atoms.
The analysis of
these Tables shows that transmutation of the nuclei of iron, of which the
cathodes are made, results in the formation of chromium and copper in both
cases. Apparently, aluminium (Fig 98, b), chlorine (Fig. 98,c) and calcium (Fig. 99) are formed from
the destroyed sodium nuclei. In any case, free protons and neutrons are formed.
But not all free
protons and neutrons are spent for the formation of the atomic nuclei of
aluminium, chlorine and calcium. A part of them is spent for the formation of
the hydrogen atoms. In any case, the atoms and the molecules of hydrogen are
formed.
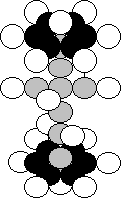
Ca
(20,20)
Fig. 99. Diagram of
the nucleus of the calcium atom
Absence of sodium
atoms on the surface of the cathode (Table 48) is an apparent feature of
destruction of the nuclei of this element during the plasma electrolytic
process. As relative quantity of the atoms of aluminium, chlorine and calcium,
which are formed and settled at the cathode, is not large, the solution of NaOH
generates more gases than the solution of KaOH (Table 44).
Another variant is
possible. When the atoms of alkali metal bombard the cathode atoms, they are
destroyed completely and destroy the atoms of the cathode materials. Under the
notion “completely” we’ll understand such state when both the atom and the
nucleus are destroyed. In this case, the protons of the destroyed nuclei begin
to form the hydrogen atoms. The process of fusion of the atoms and the
molecules of hydrogen generate additional thermal energy.
Transmutation of
the atomic nuclei of alkaline metals and the atomic nuclei of the cathode
material during plasma electrolysis of water increases the content of gases in
the gas-vapour mixture. Additional thermal energy is generated not by the
nuclear process, but the process of fusion of the atoms and the molecules of
hydrogen, which are formed from the destroyed molecules of water and from the
atomic nuclei of alkaline metals and the atomic nuclei of the cathode.
Plasma electrolytic process opens new prospects in
study of matter on the nuclear, atomic and molecular levels.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index