<<
Back to Kanarev's Physchemistry Book Index
14. WATER IS A SOURCE OF
ELECTRIC ENERGY
14.1. Common
Theoretical and experimental
investigations show that water is a source of not only thermal energy and
energy being available in hydrogen and oxygen, but a source of electric energy
as well. The diagram of water molecule with ten electrons is given in Fig. 72. We have called this structure a charge molecule
of water [75], [99], [109]. It has turned out that there is a possibility to
separate the electron, which belongs to hydrogen atom connected with the eighth
electron of the hydrogen atom, from water molecule. In this case the proton of
hydrogen atom will be connected with the second electron of oxygen atom, and
the water molecule will lose one
electron and become a semi charged one (Fig. 100).
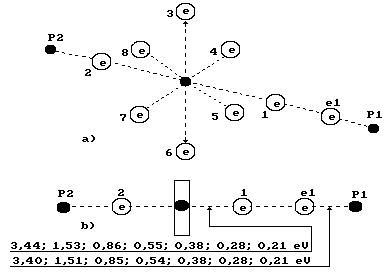
Fig. 100.
Diagram of the third model of water molecule
Quantity of electricity coulombs, which is generated
in one litre of water when each water molecule loses one electron, will be
equal to product of Avogadro constant by quantity of moles of water molecules
in one litre [109]
![]() Coulombs.
(311)
Coulombs.
(311)
Taking into consideration the fact that one ampere-hour
is 3600 coulombs of electricity, we’ll find electrical capacity of one litre of
water
![]() Ah
(312)
Ah
(312)
Experimental investigations show that electrical potential
which exceeds the potential delivered to the solution is formed in the
electrolytic solution during the definite modes of plasma electrolysis of
water. As a result, electric power, which exceeds electric power introduced
into the solution, is generated in the electrolytic solution [103].
The analysis of
binding energies between the electrons and the protons of the hydrogen atoms in
the cluster, which consists of two water molecules (Fig. 101), shows the
possibility of realization of various variants of disruption of these bonds.
Under the usual conditions the bond ![]() is broken between the
protons
is broken between the
protons ![]() and
and ![]() , which belong to the hydrogen atoms in the water molecule.
Simultaneous break of the bonds
, which belong to the hydrogen atoms in the water molecule.
Simultaneous break of the bonds ![]() and
and ![]() is possible. In the
last case, the hydrogen molecule is released. Realization of this or that
variant of the bond break depends on the temperature of the environment, in
which the water molecules are situated.
is possible. In the
last case, the hydrogen molecule is released. Realization of this or that
variant of the bond break depends on the temperature of the environment, in
which the water molecules are situated.
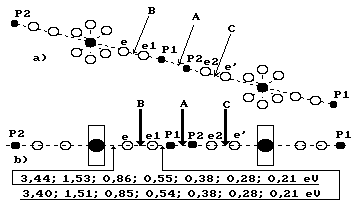
Fig. 101. Diagram
of the cluster, which consists of two water molecules
For example, if the
water molecules are in steamy state in a cloud, realization of the break ![]() will lead to the
formation of positively charged water molecules in the cloud. In another cloud,
with another temperature, the bonds break
will lead to the
formation of positively charged water molecules in the cloud. In another cloud,
with another temperature, the bonds break ![]() or
or ![]() is possible as well
as the formation of the negatively charge ions
is possible as well
as the formation of the negatively charge ions ![]() in the cloud.
in the cloud.
As realization of
this or that variant of bond break depends on the temperature, we know binding
energies and can model this process and use it for production of electric
energy from water.
14.2. Fuel Cell Efficiency
Fuel cells are
considered to be one of the most prospective consumers of hydrogen. But
efficiency of the process of the connection of hydrogen with oxygen in a fuel
element as well as the formation of electric power are studied insufficiently
[78].
The data of one of
the fuel cells are given in the report [78]. At hydrogen consumption of 2 kg
per hour it generates 30 kWh of electric power. As one cubic meter of gaseous
hydrogen weighs 90 g, 2 kg of liquid hydrogen contain 22.2 m3 of
gaseous hydrogen. If we take into consideration that in order to produce 1 m3
of hydrogen the best industrial electrolyzers consume 4 kWh and assume the energy value as 100%, we’ll get
energy efficiency of the fuel cell
![]() .
(313)
.
(313)
The source of
information [79] reports that efficiency of fuel cells of the third generation
with solid electrolyte is near 50% and the use of technology of fuel cells
allows to increase efficiency of electric power up to 75%; taking into consideration heat generated by them, efficiency
is increased by 90% or 95%.
The same source
informs that in April, 1999, the firm “Ecostar Electric Drive Systems” (joint
venture of the companies “Ford”, “Daimler-Benz “ and “Ballard Power Systems”)
has been set up for elaboration of a vehicle employing fuel cells. The total
amount of investments in the whole work has already reached 700 m dollars. This
firm intends to begin the production of series vehicles employing fuel cells in
2004 [109].
Efficiency of fuel
cells depends on efficiency of the use
of electric possibilities of hydrogen itself. If quantity of the electrons,
which belong to the atoms of hydrogen and take part in the formation of
electric power of a fuel cell, is taken
into consideration, efficiency of physical and chemical process of this cell is
less than 1%. Let us make a calculation for the fuel cell, which is described
in the report [78]. This fuel cell generates 30 kW of electric power when 2 kg
(22.2 m3) of liquid hydrogen is consumed per hour. As the mole of
gaseous hydrogen is equal to 22.4 litres, it is necessary to consume
22222.22/22.4=992.06 moles of molecular hydrogen for the production of 30 kW of
electric power [109].
We’d like to remind
that a value equal to the product of
Avogadro number N=6.022×1023 by
the electron charge ![]() = 1.602×10-19 is called Faraday constant
= 1.602×10-19 is called Faraday constant ![]() . This value is
measured in coulombs (C) per mole of
substance.
. This value is
measured in coulombs (C) per mole of
substance.
![]() C/mol. (314)
C/mol. (314)
If all protons of
992.06 moles of molecular hydrogen give
their electrons to electric net of the fuel cell, 992.06×2×96485=191437818.2
coulombs of electricity are formed, as a result. These are potential possibilities
of 22.2 m3 of molecular hydrogen. In what way are these possibilities used by
modern fuel cells?
The fuel cell being
considered operates at voltage of 100 V; that’s why when 30 kW are generated, current of 30000/100=300 amperes per
hour circulates in its electric circuit. 3600 coulombs of electricity are consumed
at 1 ampere/hour and 1080000.0 coulombs are consumed at 300 ampere per hour. If
we assume that potential quantity of coulombs
of electricity, which 22.2 m3 of hydrogen contain (191437818.2 coulombs)is 100%,
actual quantity of coulombs of electricity generated by the fuel cell is
![]() (315)
(315)
These are the main reserves of efficiency improvement
of the fuel cells!
Feeding of
molecular hydrogen to the fuel element is the main cause of a very low (0.57%)
electrical efficiency of the fuel cell. There is every reason to hope that
minimal tenfold improvement of this efficiency will take place in the nearest
future.
The specialists,
who are busy with the investigation of
fuel cells, should pay attention to importance of the analysis of water
produced in the results of its operation. We have shown that water molecules
can contain both all 10 electrons (charged water) and 8 electron (discharged
water). If water is pure (without impurities), there should be a difference in
weight of one litre of charged water and discharged water, which can be easily
found out. The greater the number of discharge molecules in water being
produced after the operation of the fuel cell, the more effective the energy
possibilities of hydrogen are used.
The given
calculations show that energy properties of hydrogen in fuel cells are used
only by 0.6%. Tenfold increase of this
index is equal to the transit to hydrogen power in all field of human activities
[41].
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index