<<
Back to Kanarev's Physchemistry Book Index
12.5. Results of Fine Plasma
Experiments
If water molecules are
in ac filed and in high temperature thermal field at the same time during
active turbulent flow, the process of separation of the protons and the
hydrogen atoms from water molecules becomes chaotic. In this case, sundry
variants of this process are possible. The separation of hydrogen atoms from
water molecules is the most probable variant (Fig. 92, a, b).
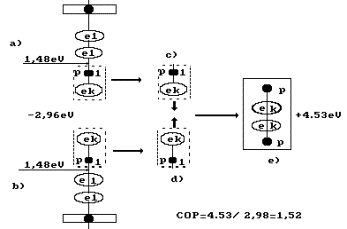
Fig. 92. Diagram of
hydrogen molecule fusion during water electrolysis: a), b) - water molecules;
c),
d) - hydrogen atoms; e) -
orthohydrogen molecule
As it is clear, 4.53 eV of energy is released
during fusion of one hydrogen molecule (Fig. 92). In this case, the following
reaction takes place at the cathode
![]() .
(299)
.
(299)
During plasma water
electrolysis, the oxygen formation process in the anode area is less intensive
than during low-voltage electrolysis, because its greater part is released in
the cathode area together with hydrogen. If the bonds of the oxygen atom with
the hydrogen atoms are destroyed in the water molecule only in a thermal way,
no additional thermal energy is generated (as we have shown earlier). That is
why the thermal energy efficiency index of such process will be as follows (Table 41) [109].
![]() (300)
(300)
Table 41
|
Indices |
1 |
2 |
3 |
Mean |
|
1 – mass of the solution,
which has passed through the reactor m,
gr. |
1100 |
1070 |
1060 |
1077 |
|
2 – temperature of solution
at the input of the reactor t1,
degrees |
17 |
17 |
17 |
17 |
|
3 – temperature of the
solution at the output of the reactor t2, degrees |
22 |
22 |
22 |
22 |
|
4 – temperature difference
of the solution Dt= t2
- t1, degrees |
5 |
5 |
5 |
5 |
|
5 – durability of the
experiment Dt, s |
300 |
300 |
300 |
300 |
|
6 – number of rotations of
the disc of the counter during the experiment n, rotations |
2.4 |
2.4 |
2.4 |
2.4 |
|
7 – electric power consumption
according to the reading of the counter, Note: 600 rotations of the
counter correspond to 1 kWh of electric power |
14.4 |
14.4 |
14.54 |
14.4 |
|
8 – reading of voltmeter V,
V |
140 |
140 |
140 |
140 |
|
9 – reading of ammeter I, A |
0.34 |
0.34 |
0.34 |
0.34 |
|
10 – electric power
consumption according to indices of voltmeter and ammeters, E2=I×V×Dt, kJ |
14.28 |
14.28 |
14.28 |
14.28 |
|
11 – power spent for heating
of the solution, E3=C×m×Dt, kJ |
23.45 |
22.42 |
22.21 |
22.69 |
|
12 – reactor efficacy
efficiency index according to the reading of the counter K |
1.60 |
1.56 |
1.54 |
1.57 |
|
13 – reactor efficiency index
according to the reading of voltmeter and ammeter K2= E3/
E2 |
1.64 |
1.57 |
1.56 |
1.59 |
Let us consider one more variant of hydrogen
molecule formation from a destroyed water molecule. It is clear from Fig. 91 a, b, c that in order to separate the proton of the hydrogen atom from
water molecule, it is necessary to spend 1.48 eV of energy. Later on we’ll show that during further fusion of two
hydrogen atoms (0.86x2)=1.72 eV of energy will be released. Then during
hydrogen molecule fusion 4.53 eV of energy will be released. During the fusion
process of two hydrogen atoms and a hydrogen molecule, total quantity of energy
will be 1.72+4.53=6.25 eV. The following reaction will take place at the
cathode at that time [109]:
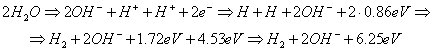 (301)
(301)
where H+
is the proton.
In this case, the index of heat energy efficacy will be equal
to (Fig. 93), (Table 42) [109]
K= 6.25/2.96=2.11. (302)
A modified
plasma-electrolytic reactor (Fig. 88) adjusted to non-plasma operation mode has
been used for the experiments (Fig. 93, Tabl. 42). The experiments method is
simple: electrolytic solution passes through an electrolytic cell (reactor).
Released energy has been determined according to difference of temperature at
the input and at the output of the reactor and expended energy has been
determined with the help of a domestic electricity meter as well as voltmeter
and ammeter of the highest accuracy class. Energy losses have not been taken
into consideration, but one has
tried to make temperature difference ![]() a small one in order to reduce them.
a small one in order to reduce them.
Let us give the second variant of the
calculation of the experimental result (Table 42) using not the theoretical
results of energy consumption for hydrogen production, but the experimental
ones. One cubic metre of hydrogen contains 1000/22.4=44.64 moles of molecular hydrogen
or 89.28 moles of monatomic hydrogen. During fusion of one atom of hydrogen
0.86 eV of energy is released; during fusion of 89.28 moles of hydrogen atoms
the following quantity will be released
![]() (303)
(303)
Further fusion of one cubic metre of
hydrogen will add
![]() . (304)
. (304)
The results of experimental check of the given theoretical calculation are given
in Table 42.
Table 42
|
Indices |
1 |
2 |
3 |
Mean |
|
1 – mass of the solution, which has passed through the reactor m,
degrees |
1200 |
1230 |
1160 |
1197 |
|
2 – temperature of solution at the input of the reactor t1, degrees |
20 |
20 |
20 |
20 |
|
3 – temperature of the solution at the output of the reactor t2,
degrees |
31.0 |
30.5 |
31.0 |
30.8 |
|
4 – temperature difference of
the solution Dt= t2 - t1,
degrees |
11.0 |
10.5 |
11.0 |
10.8 |
|
5 – durability of the exper. Dt, s |
300 |
300 |
300 |
300 |
|
6 – number of rotations of the disc of the counter during the
experiment n, rotations |
4.44 |
4.44 |
4.44 |
4.44 |
|
7 – electric power consumption according to the reading of the counter
E1= nЧ3600/600 kJ Note: 600 rotations of the counter correspond to 1 kWh of electric
power |
26.64 |
26.64 |
26.64 |
26.64 |
|
8 – reading of voltmeter V, V |
40 |
40 |
40 |
40 |
|
9 – reading of ammeter I, A |
1.80 |
1.80 |
1.80 |
1.80 |
|
10 – electric power consumption according to indices of voltmeter and
ammeters, E2=IЧVЧDt, kJ |
21.60 |
21.60 |
21.60 |
21.60 |
|
11 – power spent for heating of the solution, E3=CЧmЧDt, kJ |
55.31 |
54.11 |
53.46 |
54.29 |
|
12 – reactor efficacy efficiency index according to the reading of the
counter K1= E3/ E2 |
2.08 |
2.03 |
2.01 |
2.04 |
|
13 – reactor efficiency index according to the reading of voltmeter
and ammeter K2= E3/ E2 |
2.56 |
2.50 |
2.47 |
2.51 |
Fig.
93. Diagram of fusion of an atom and a molecule of hydrogen in the process of
water electrolysis: a), b) - water molecules; c), d) - hydrogen atoms; e) –
orthohydrogen molecule
If we add together the energies of fusion of
the atoms and the molecules of hydrogen, we’ll get (7322.3+19463.0)=26785.3 kJ.
In order to produce one cubic meter of hydrogen according to the existing
technology, it is necessary to spend (4.0x3600)=14400 kJ. Index K of heat
energy efficacy of such process of electrolysis will be (Table 42) [109]
К = (26785.3/14400)= 1.86. (305)
If we add energy content of hydrogen being produced
(90x142)=12780 kJ, index ![]() of total energy efficacy will be [109]
of total energy efficacy will be [109]
![]() = (39565.3/14400)=2.75. (306)
= (39565.3/14400)=2.75. (306)
During the analysis
of plasma electrolytic process, one should take into consideration the fact
that in some operation modes water in the cathode area is decomposed not only
into hydrogen and hydroxyl ![]() , but into hydrogen and oxygen. In this case, ion
, but into hydrogen and oxygen. In this case, ion ![]() is decomposed. We
have shown that electrodynamic binding energy of the hydrogen atom with the
oxygen atom in water molecule is equal to 0.74 eV, and thermal binding energy
is 1.48 eV.
is decomposed. We
have shown that electrodynamic binding energy of the hydrogen atom with the
oxygen atom in water molecule is equal to 0.74 eV, and thermal binding energy
is 1.48 eV.
If in the cathode zone only the thermal process of
water molecule decomposition into hydrogen and oxygen took place, the energy
expenses for this process during fusion of one cubic meter of hydrogen would be
![]() (307)
(307)
If the water molecules were decomposed
into hydrogen and oxygen in the cathode area only mechanically, the energy
expenses would be half, i.e. 6364 kJ.
Here, the problem is in reduction of intensity of the process of
repeated bonding of hydrogen and oxygen in the plasma area. If this problem is
not solved, energy efficacy of this process is increased, because during water
molecules fusion the energy volume will be twice as much than spent for their
mechanical destruction.
In reality, the
protons and the atoms of hydrogen are be separated from water molecule
simultaneously with the process of destruction of water molecule into hydrogen
and hydroxyl OH- and into hydrogen and oxygen; that’s why thermal
energy efficacy index will vary within the range of 1.10…2.00 (Table 41, 42).
12.6.
Plasma-electrolytic Reactor as Gas Generator
The new theory of water electrolysis predicts the
possibility of significant reduction of power consumption for production of
hydrogen from water.
But it is possible to
do it if the above-mentioned conditions are observed. For example, let us pay
attention to the orthohydrogen structure, which diagram is given in Fig. 53, b. This structure
is formed when the hydrogen atoms of two water molecules approach each other.
In this case, each of two water molecules gives one proton and one electron to
the hydrogen molecule, and the hydrogen molecule is formed without the
electrons emitted by the cathode, i.e. without direct consumption of electric
energy for this process. In this case, electric energy is spent only for a
separation of the hydrogen molecule being formed. Two water molecules connected
in such a way correspond to the simplest cluster [109].
High temperature of
plasma forms the conditions when a set of various processes takes place at the
cathode. First of all, water is boiled and evaporated. At the same tome, one
part of water molecules is disintegrated with a release of the atomic hydrogen,
another part of the molecules forms the orthohydrogen molecules. A part of
water molecules is disintegrated completely and is released at the cathode
together with hydrogen and oxygen. A part of hydrogen is combined with oxygen
again generating micro explosions (noise) and forming water.
But one should bear
in mind that if plasma disintegrates water molecule into hydrogen and
oxygen and if these gases contact
plasma, hydrogen is combined with oxygen, and water is formed. Noise generated
by plasma is hydrogen micro explosions. Taking into consideration the
above-mentioned fact the larger the volume of hydrogen burnt in plasma, the
smaller its volume in the gas-vapour mixture. It means that such reactor
operation modes are required when quantity of burnt hydrogen is minimal one.
During plasma
electrolysis of water, water vapor, hydrogen and oxygen are released
simultaneously. If vapor is condensed, gas mixture is released (Fig. 94).
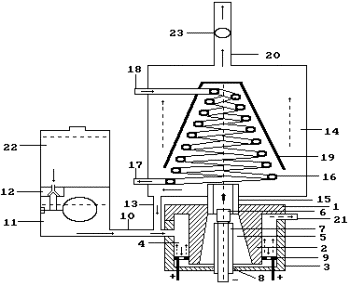
Fig. 94. Diagram of
the plasma electrolytic generator of hydrogen: 1 - lid of the reactor; 3 - body
of the reactor; 6 - the cathode; 9 - the anode; 11 - solution doser; 16 -
cooler; 20 - pipe for gas release; 23 –
anemometer
In order to measure
gas flow rate, both a conventional anemometer and an electronic one have been
used. Diameter of the electronic anemometer was equal to internal diameter of
the gas make tube (23, Fig. 94). Its readings were registered and processed by
the computer. The experiment was performed dozen time, and each time its
readings were reproduced with small deviations [109]. But we had no hydrogen
analyzer, that’s why the results being obtained cannot be considered as final
ones. We admonished it in all editions of the book “Water
is a New Source of Energy” with such a phrase: “We abstain from lending an
official status to these results with the hope to get necessary financing and
to repeat them with a complete set of the necessary devices” [109, page 176].
In the middle of
the year of 2002 we received small financing, which allowed us to make a new
reactor and to buy some measuring instruments, in particular the scales with
the measurement limit up to 600 g and accuracy of 0.02 g. Careful preparation
allowed us to increase duration of continuous operation of the reactor and to
register solution consumption for gas production.
The main difficulty
of operation with the hydrogen is in the fact that its mixture with air (4-74)%
or oxygen (4-94)% is combustible, and the fact was emphasized more than once
during the experiments making the researches be very careful. The second
difficulty during hydrogen quantity measurements generated by the plasma
electrolytic reactor is in the fact that its molecule has the smallest
dimensions, that’s why it penetrates easily to the places where the molecules
of other substances do not penetrate. Molecular hydrogen diffuses easily even
into metals [39]. For example, one volume of palladium absorbs up to 800
volumes of hydrogen.
Gas flow speed was
measured with the help of various anemometers, its readings being registered
with the help of the computer. Numerous measurements and numerous analysis of
gas flow speed measurement accuracy with the help of the anemometers showed
that error of a conventional anemometer can be
100%. That’s why in order to increase safety of the experiment,
registered speed of gas flow was reduced 2fold. Taking it into consideration,
energy consumption per cubic meter of gas mixture is given in Table 43. The
given data were obtained by us together with A.I. Tlishev, D.V. Korneev and
D.A. Bebko. Durability of one repetition of the experiment is 300 s.
Table 43. Influence
of voltage on volume of gases being generated
|
U, volts |
180 |
200 |
220 |
240 |
260 |
280 |
|
W, litre |
23.2 |
81.0 |
108.0 |
127.5 |
127.5 |
121.5 |
|
|
1.04 |
0.22 |
0.14 |
0.14 |
0.15 |
0.13 |
Durable continuous operation
(10 hours) of the reactor with various solutions gave the following results
(Table 44).
Table 44.
Experimental results
|
Indices |
Water consumption, kg |
Volume of gases, |
Energy expenses, |
|
KOH |
0.035 |
8.00 |
0.52 |
|
NaOH |
0.072 |
11.20 |
0.30 |
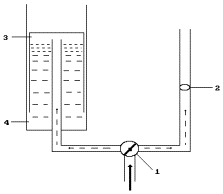
Fig. 95. Diagram of
measurement of flow rate of the gas and its volume: 1 - tap for gas flow
movement
direction
switching, 2 – anemometer, 3 – graduated tank, 4 – water tank
It is known that it
is possible to produce 1220 litres of hydrogen and 622 litres of oxygen from
one litre of water. Quantity of the gases generated by the plasma electrolytic
process is much greater than it is possible to get from consumed water (Table 44). It was a strong
reason for a search of the measurement error. For this purpose, the diagram of
measurement of flow rate of the gases and their quantity was used (Fig.
95).
The results of the
measurements were as follows. The anemometer showed that 200 litres of gas
mixture penetrated through it during 10 minutes. Nearly one litre of gases was
in the graduated tank during this period.
Thus, the
measurement of gas flow with the help of the anemometers distorted the result
200fold. It should be mentioned that the reactor operated in the production
mode of hydrogen and oxygen in the cathode zone. As a result, their mixture
burst. The pulses of these explosions increased the readings of the anemometer.
It has become
necessary to return to the reactor operation modes when few oxygen is released
in the cathode zone or to the operation with the plasma in the centre of
electric field in solution between cathode and anode. The results of these
experiments will be published in the third edition of this book. Now we have
the results of low-current
Electrolysis
of the water (Table 45).
PROTOCOL
of tests of the first model of low-current
Electrolyzers
It is known that it is
possible to produce 1.22 l of![]() + 0.622
+ 0.622 ![]() = 1.843 (
= 1.843 (![]() ) from 1 ml of
) from 1 ml of ![]() .
.
Table 45. Experimental results
|
Indices |
1 |
2 |
3 |
Average |
|
1-duration
of experiment, hour |
1 |
1 |
1 |
1 |
|
2-voltage,
V |
70 |
70 |
70 |
70 |
|
3-current, A |
0.038 |
0.080 |
0.098 |
0.072 |
|
4
–power, W |
2.7 |
5.60 |
6.44 |
4.91 |
|
5-volume
of consumed solution, ml |
1.67 |
3.98 |
4.32 |
3.32 |
|
6-volume
of the gas mixture being produced, l |
3.08 |
7.16 |
7.95 |
5.95 |
|
7-volume
of hydrogen being produced, l |
2.04 |
4.75 |
5.27 |
4.02 |
|
8-energy
consumption per l of hydrogen, W×h/l |
1.32 |
1.18 |
1.22 |
1.24 |
|
9-energy
consumption per m3 of hydrogen, kWh/m3 |
1.32 |
1.18 |
1.22 |
1.24 |
|
9-existing
energy consumption for production of 1 m3 of hydrogen from water,
kWh/m3 |
4.00 |
4.00 |
4.00 |
4.00 |
We used the weigh method too
and had received the same results. Thus
the low-current electrolysis allow us
to get the inexpensive hydrogen from
water too.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index