<<
Back to Kanarev's Physchemistry Book Index
11.3. New Theory of low voltage of Process
of Water Electrolysis
Having the obtained
information at our disposal let us begin the search of a new structure of water
molecule and a new theory of the process of its electrolysis. This theory
should eliminate the existing contradictions in the description of water
electrolysis and give replies to the following
fundamental questions:
1 - why do the
theoretical calculations demonstrate availability of additional energy during
water electrolysis and why do the existing industrial electrolyzers fail to
generate it?
2 - why do the
existing theoretical values (264-265) of binding energies of hydrogen atoms in
water molecules fail to correspond to experimental values of these energies
during water electrolysis?
First of all, in
order to find replies for these questions it is necessary to have a theory,
which would allow to calculate energies of chemical bonds of the electrons with
atomic nuclei when they are at any energy level. As the atoms of hydrogen and
oxygen play the main role during water electrolysis, we’ll determine binding
energies of their electrons with atomic nuclei. We have given some of these
calculations, but as they are very important, we’ll give them once more having
added a new information.
Taking into
consideration that ionization energy ![]() of hydrogen atom is equal to binding energy
of hydrogen atom is equal to binding energy ![]() of the electron with
the nucleus corresponding to the first energy level
of the electron with
the nucleus corresponding to the first energy level ![]() and using formulas
(212) and (213) we’ll get energies of the photons
and using formulas
(212) and (213) we’ll get energies of the photons ![]() emitted or absorbed
by the electron, and binding energies
emitted or absorbed
by the electron, and binding energies ![]() of the electron with
the atomic nucleus corresponding to
of the electron with
the atomic nucleus corresponding to ![]() energy levels (Table
35).
energy levels (Table
35).
Table 35. Spectrum
of hydrogen atom
|
Value |
n |
2 |
3 |
4 |
5 |
6 |
|
|
eV |
10.20 |
12.09 |
12.75 |
13.05 |
13.22 |
|
|
eV |
10.198 |
12.087 |
12.748 |
13.054 |
13.22 |
|
|
eV |
3.40 |
1.51 |
0.85 |
0.54 |
0.38 |
As it is clear
(Table 35), there are no energies ![]() which are obtained by
the chemists in their calculations (266-269) in the row of binding energies of
the electron with the nucleus of hydrogen atom. But energies similar to
experimental value (1.59 eV) by which gases are released during water
electrolysis are available in the row of binding energies of the electron of
hydrogen atom (1.51 eV) (Table 35) and the eighth electron of oxygen atom (1.53
eV) (Table 36). These energies correspond to the existence of the electrons on
the third energy levels.
which are obtained by
the chemists in their calculations (266-269) in the row of binding energies of
the electron with the nucleus of hydrogen atom. But energies similar to
experimental value (1.59 eV) by which gases are released during water
electrolysis are available in the row of binding energies of the electron of
hydrogen atom (1.51 eV) (Table 35) and the eighth electron of oxygen atom (1.53
eV) (Table 36). These energies correspond to the existence of the electrons on
the third energy levels.
Thus, among binding
energies of the electron of hydrogen atom with its nucleus there are
energies (1.51eV) similar to
experimental value (1.59eV). Let us
determine the same energies for the oxygen atom electrons.
As the surface
electrons of the atoms take part in
chemical reactions namely, let us consider only the calculation of energies ![]() , the absorbed and emitted photons as well as binding
energies
, the absorbed and emitted photons as well as binding
energies ![]() of the electrons with
the nuclei of the first two surface electrons of oxygen atom.
of the electrons with
the nuclei of the first two surface electrons of oxygen atom.
Ionization energy
of the first electron of oxygen atom is equal to ![]()
![]() =13.618 eV, and
its binding energy with the atomic nucleus corresponding to the first energy
level is equal to
=13.618 eV, and
its binding energy with the atomic nucleus corresponding to the first energy
level is equal to ![]() =13.752 eV. Energy indices calculation of this electron
according to the formulas (212) and (213) gives the following results (Table
36).
=13.752 eV. Energy indices calculation of this electron
according to the formulas (212) and (213) gives the following results (Table
36).
Table 36. Spectrum
of the 1th electron of oxygen atom
|
Value |
n |
2 |
3 |
4 |
5 |
6 |
|
|
eV |
10.18 |
12.09 |
12.76 |
13.07 |
13.24 |
|
|
eV |
10.16 |
12.09 |
12.76 |
13.07 |
13.24 |
|
|
eV |
3.44 |
1.53 |
0.86 |
0.55 |
0.38 |
As it is clear,
binding energies of the first electron of oxygen atom (Table 36) practically
coincide with the corresponding binding energies of the electron of hydrogen
atom (Table 35). The energy corresponding to the third level (1.53eV) is
similar to experimental value of gas release energy (1.59 eV) during water
electrolysis. Theoretical values of binding energies of the electron of the
first atom of hydrogen and the first electron of oxygen atom in water
molecule obtained on the grounds of
spectrum formation law (212) and (213) are similar to the experimental values
of this energy.
Now we have every
reason to suppose that the first
electron of oxygen atom establishing the bond with the first atom of
hydrogen in water molecule is on the third (![]() ) energy level (Table 36).
) energy level (Table 36).
When we
have analysed the regularity of the change of binding energies of the electrons
of the oxygen atom and other atoms with their nuclei, we have found that they
have almost equal binding energies with the atomic nuclei in case all electrons
are present in the atom. That’s why we’ll consider that water molecule symmetry
provides equal binding energies with the nucleus of its first electron and the
second one.
Thus, we have
removed the second contradiction between the theory and the experiment in water
electrolysis. Now experimental value of binding energy of the electron of hydrogen atom with the first electron of
oxygen atom in water molecule coincides with theoretical value of this energy.
Low voltage process of water electrolysis takes place when
voltage is (1.6-2.3) V and strength of current of hundreds of amperes. Large
strength of current proves large expenditure of electrons. As the first
electron of the oxygen atom is situated from its nucleus at the longest distance
than other electrons, the proton of the hydrogen atom, which is bond with this
electron, is the first to come nearer to the cathode and gets the electron ![]() from it (Fig. 78, a). After each of
two water molecules gets the electron
from it (Fig. 78, a). After each of
two water molecules gets the electron ![]() , their surface electrons are united at once and form a
cluster of two water molecules (Fig. 78, a, b), which are connected by two electrons
, their surface electrons are united at once and form a
cluster of two water molecules (Fig. 78, a, b), which are connected by two electrons ![]() emitted by
the cathode. As it is clear, the orthohydrogen molecule is in the chain of the protons and the electrons, which unite
two water molecules (Fig. 78, a, b). As the electrons, which have arrived from the
cathode, have passed the free state phase, hydrogen molecule fusion in this
chain is accompanied by release of energy.
emitted by
the cathode. As it is clear, the orthohydrogen molecule is in the chain of the protons and the electrons, which unite
two water molecules (Fig. 78, a, b). As the electrons, which have arrived from the
cathode, have passed the free state phase, hydrogen molecule fusion in this
chain is accompanied by release of energy.
It is clear from
Fig. 78 that two electrons
![]() emitted by the
cathode are used for the formation of one hydrogen molecule. In accordance with
Faraday’s law, in this case two faraday coulombs of electricity are used for
the formation of one mole of hydrogen 2F=2×96485=192980 or
192980/3600=53.60 A×h/mol. If
electrolysis takes place at voltage of 1.70 V, for production of one mole of
hydrogen E=I×V=53.6×1.70=91.12 W×h will be spent,
and for production of 1
emitted by the
cathode are used for the formation of one hydrogen molecule. In accordance with
Faraday’s law, in this case two faraday coulombs of electricity are used for
the formation of one mole of hydrogen 2F=2×96485=192980 or
192980/3600=53.60 A×h/mol. If
electrolysis takes place at voltage of 1.70 V, for production of one mole of
hydrogen E=I×V=53.6×1.70=91.12 W×h will be spent,
and for production of 1 ![]() E=(1000/22.4)×91.12=1476 kJ/
E=(1000/22.4)×91.12=1476 kJ/![]() =4.10 kWh will be spent.
=4.10 kWh will be spent.
As it is clear, the
calculations with the use of Faraday’s law give a result, which coincides with
the experiment almost completely. If hydrogen formation were accompanied by the
process of fusion of its molecules, energy would be released
![]()
If
we take into account only the hydrogen molecule fusion and do not take into
account the oxygen molecule fusion, heat energy efficacy index should be as
follows:
![]() .
.
But it is well
known that total heat energy efficacy index of modern electrolysers is less than unit.
Why? We’ll try to find an answer to this question (Fig. 78).
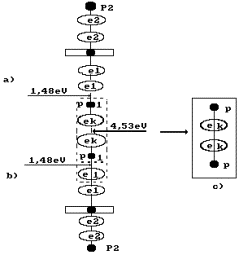
Fig. 78. Diagram of
formation of the orthogydrogen molecule (s. Fig. 53, a)
Fusion energy of one mole of the hydrogen molecules is
equal to 436 kJ. Let us convert this energy in electron volts in the
calculation per molecule [109].
![]() . (271)
. (271)
The value of this energy is shown to the right of the hydrogen molecule
situated in a cluster chain (Fig. 78). To the left, binding energies 1.48 eV of
the hydrogen atoms with the oxygen atoms in water molecule are shown. Energy
4.53 eV of fusion of the hydrogen molecule redistributes binding energies in
the cluster chain in such a way that binding energies 1.48 eV of the hydrogen
atoms with the oxygen atoms in water molecules become equal to zero, and the
orthohydrigen molecule is separated from the cluster chain (Fig. 78, c).
Thus, the difference
between energy 4.53 eV of fusion of the hydrogen molecule and total binding
energy (1.48+1.48)=2.96 eV becomes equal to (4.53-2.96)=1.57 eV. This energy is
spent to heating the electrolytic solution.
That’s why during 1![]() of hydrogen is released not 44.64x436=19463 kJ will be
released, but the following quantity of energy
of hydrogen is released not 44.64x436=19463 kJ will be
released, but the following quantity of energy
![]() (272)
(272)
Near the cathode the following chemical reaction will take
place
![]() (273)
(273)
It is natural
that quantity of heat energy 4058 kJ is a part of total energy 14760 kJ, which
is spent for production of one cubic metre of
hydrogen [109]. Thermal effectiveness index of this process is
![]() (274)
(274)
If we take into
account that energy content of one gram of hydrogen is equal to 142 kJ and
cubic metre of this gas weighs 90 g, total energy effectiveness index will be
![]() (275)
(275)
We should note that
we have not taken into consideration energy content of oxygen being obtained,
because oxygen from the air is usually used when burning hydrogen.
Other variants of
hydrogen molecules formation are possible. For example, let us pay attention to
the structure of orthohydrogen, which is shown in Fig. 53, b. This structure is
formed when the first hydrogen atoms of two water molecules of the first model
come nearer to each other (Fig. 72) without the electron emitted by the
cathode, i.e. without direct consumption of electric energy for hydrogen
molecule formation (Fig. 79). According to Faraday’s law, energy consumption
for such hydrogen
molecule formation process is equal to zero.
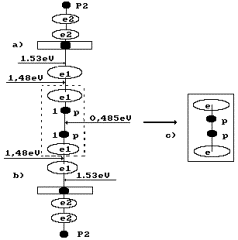
Fig. 79. Diagram of
formation of the second model of
orthohydrogen (s. Fig. 53, b); a) and
b) of the diagram
of water molecules; c) orthohydrogen
In this case, each
of two water molecules gives one proton and one electron to the hydrogen
molecule, and the hydrogen molecule is formed without electrons emitted by the
cathode, i.e. without direct expenses of electric power for this process. In
this case, electric power is spent only for the separation of the hydrogen
molecule being formed from two water molecules. Energy consumption for production of one cubic meter of hydrogen
is as follows:
![]() (276)
(276)
One cubic metre of
hydrogen weighs 90 g. Energy content of one gram of hydrogen is equal to 142
kJ. When this hydrogen is burnt, energy of 90×142=12780 kJ will be released.
Total index ![]() of energy efficacy of
the process will be as follows:
of energy efficacy of
the process will be as follows:
![]() (277)
(277)
If
hydrogen were released as a result of fusion of its molecules, energy would be
generated [109]:
![]() (278)
(278)
Electrolyzers consume 4 kWh of electric
power or (3600x4)=14400 kJ in order to produce one cubic meter of hydrogen.
Taking into consideration energy (19463.0) of fusion of one cubic meter of
hydrogen and energy (14400) spent for its production, we’ll find the index of water electrolysis process
efficacy [109]
![]() . (279)
. (279)
Now let us consider how a parahydrogen molecule is formed
(Fig. 53, c). Electron ![]() emitted by the cathode
(Fig. 80) combines two molecules
of water. There is a hydrogen molecule structure in the chain being formed. It
is formed by the proton of the hydrogen atom of one water molecule, the
electron
emitted by the cathode
(Fig. 80) combines two molecules
of water. There is a hydrogen molecule structure in the chain being formed. It
is formed by the proton of the hydrogen atom of one water molecule, the
electron ![]() emitted by the
cathode and the proton with its electron (the hydrogen atom) of the second
water molecule. Thus, one electron emitted by the cathode is spent for the
formation of one parahydrogen molecule.
emitted by the
cathode and the proton with its electron (the hydrogen atom) of the second
water molecule. Thus, one electron emitted by the cathode is spent for the
formation of one parahydrogen molecule.
As
only one electron is spent for formation of the parahydrogen molecule shown in
Fig. 80, according to Farady’s law energy
expenses for such process should be half. The calculation according to our
method gives an actual result (276).
If the
hydrogen molecules are formed according to the diagrams shown in Fig. 78, 79
and 80, there are no hydrogen atom protons and hydrogen atoms in free state in the solution. There are no
processes of fusion of the atoms and the hydrogen atoms, there is no additional
energy, which corresponds to these processes. An index ![]() of energy efficacy of such processes of water electrolysis
will be less than one.
of energy efficacy of such processes of water electrolysis
will be less than one.
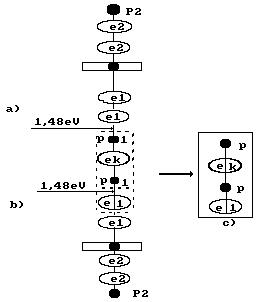
Fig. 80. Diagram of parahydrogen molecule formation (s. Fig. 53, c):
and b) water molecules; c) parahydrogen molecule
Let us consider the
reactions, which take place near the anode. It is known that hydroxyl ion (Fig.
75) having the
negative charge ![]() moves to the anode (Fig. 81, a). Two hydroxyl ions give
one electron each to the anode, connect with each other and form hydrogen peroxide
moves to the anode (Fig. 81, a). Two hydroxyl ions give
one electron each to the anode, connect with each other and form hydrogen peroxide
![]() (Fig. 81, b).
(Fig. 81, b).
It is known that
the process of formation of hydrogen peroxide is an endothermic one, and the
process of formation of oxygen molecule is an exothermic one. When one cubic
metre of hydrogen is produced, the process of formation of hydrogen peroxide
requires 22.32 x 109.00= 2432.88 kJ. Due to it, even during plasmaelectrolytic
process the temperature of the solution in the anode area remains low.
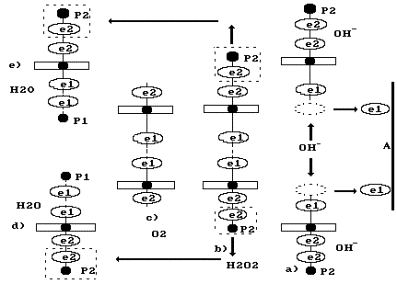
Fig. 81. Diagrams:
a) transfer of electrons e1 by the ions ![]() to the anode A; b) formation of
hydrogen peroxide
to the anode A; b) formation of
hydrogen peroxide ![]() ; c) formation of oxygen molecule
; c) formation of oxygen molecule ![]() and two molecules of water d)
and e)
and two molecules of water d)
and e)
If the oxygen
molecule fusion process existed, 22.32x495.00=11048.40 kJ would be released during
production of one cubic metre of hydrogen in the anode area. If we subtract
energy absorbed during hydrogen peroxide fusion from this value, we’ll get
11048.40-2432.88=8615.52 kJ. If we add this energy to hydrogen molecule fusion
energy of 19463.00 kJ, we’ll get 28078.52 kJ. In this case total index of
energy efficacy should be as follows: 28078.52/14400=1.95. As this energy does
not exist in reality, this fact confirms a hypothesis concerning the lack of
the hydrogen molecule fusion process in the cathode area and the oxygen
molecules in the anode area. The hydrogen molecule (Figs 78, 79 and 80) and the oxygen
molecule (Fig. 81,
b) are formed prior to a release into the free state, that’s why energy of
their fusion is not generated.
When two electrons
are transferred to the anode by two ions of hydroxyl (Fig. 81, a), a hydrogen
peroxide molecule is formed (Fig. 81, b), which decomposes and forms an oxygen molecule
(Fig. 81, c) and two
hydrogen atoms; they are combined with the hydroxyl ions and form two water
molecules (Fig. 81
d, e) If we take it into account, the chemical reaction in the anode area will
be written in such a way:
![]() (280)
(280)
Thus, we have
removed the contradictions of the existing theory of low-voltage process of
water electrolysis and have worked out a new theory, which describes this
process in detail and reflects reality more accurately.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index