<<
Back to Kanarev's Physchemistry Book Index
11.2. Models of Water Molecules and
its Ions
Water can
demonstrate a variety of properties. The possibilities of this variety are
available due to the differences of water molecule structure. The information
obtained by us allows to discover and analyses the structural peculiarities of
water molecule. We have shown that the electrons in the atom have no orbital
movement, they interact with the nucleus like a rotating whipping top. As there
are the electrons and the protons of the like electrical fields and magnetic
ones with vividly expressed magnetic poles in the structure, it gives them the
possibility to interact with each other and to limit their rapprochement. Due
to this fact the bond between the valence electrons in the molecule and between
the electrons and the protons in the atom can be depicted with the help of simple
lines.
We have already
noted that the bonds between the atoms in the molecule form the surface
electrons, which we call valence electrons. Valence electrons of the atoms,
which form a molecule, can get connected with each other or with the protons of
the nuclei if the proton cell is free.
Hitherto, the water
molecule models are depicted in such a way that the angle between the hydrogen
atoms is 105![]() [46], [58], [109]. We do not know the way this value has
been derived. But if we suppose that it corresponds to reality (we have doubt in it), the water molecule model will
be such as it is shown in Fig. 71 taking into consideration the model of the atomic
nucleus of oxygen (Fig. 28). This model gives the reason to believe that the electrostatic
repulsive forces operating between the first (e1, P1) and the second (e2, P2)
hydrogen atoms increase the angle between them up to 105
[46], [58], [109]. We do not know the way this value has
been derived. But if we suppose that it corresponds to reality (we have doubt in it), the water molecule model will
be such as it is shown in Fig. 71 taking into consideration the model of the atomic
nucleus of oxygen (Fig. 28). This model gives the reason to believe that the electrostatic
repulsive forces operating between the first (e1, P1) and the second (e2, P2)
hydrogen atoms increase the angle between them up to 105![]() . But this model does not explain the reason of water
expansion during freezing. If we imagine that the hydrogen atoms are connected
with the axis electrons of the oxygen atom (Fig. 72), the reason of water
expansion during its freezing can be explained.
. But this model does not explain the reason of water
expansion during freezing. If we imagine that the hydrogen atoms are connected
with the axis electrons of the oxygen atom (Fig. 72), the reason of water
expansion during its freezing can be explained.
As during cooling
the electrons emit photons and approach the atomic nucleus, six ring electrons
of the oxygen atom in water molecule (Fig. 72) approach the atomic nucleus
and remove the axial electrons from the nucleus by their static field. In this
case the distance between the hydrogen atoms arranged on the water molecule
axis is increased. Due to it, the length of the bond with the neighbouring
water molecules is increased during its freezing. Taking it into consideration
we prefer the water molecule model shown in Fig. 72, and we’ll use this model
only in the future.
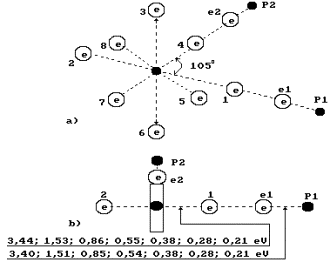
Fig. 71. Water molecule
structure with the angled of 105![]() between the hydrogen atoms
between the hydrogen atoms
The structure of
hydrogen atom (Fig. 50) demonstrates that if this atom unites with the first
electron of oxygen atom by its only electron, the proton will be on the surface
of the molecule and will form a zone with positive charge, which is generated
by the proton of hydrogen atom (Fig. 72). The proton of the second hydrogen
atom forms the same zone. It is connected with the second electron of oxygen
atom (Fig. 72).
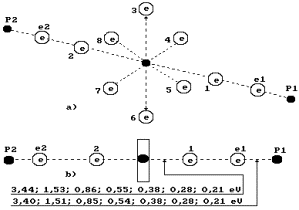
Fig. 72. Diagram of
the first (charged) model of water molecule:
1, 2, 3, 4, 5, 6, 7, 8 are the
numbers of the electrons of oxygen atom; P is the nuclei of hydrogen atoms
(protons); ![]() and
and ![]() are the numbers of
hydrogen electrons.
are the numbers of
hydrogen electrons.
The negatively
charged zone is formed by the oxygen atom electrons arranged on a ring round
the oxygen atom axis [2], [54], [55], [58]. Let us pay attention to the fact
that binding energies between the proton ![]() and the
electron
and the
electron ![]() (Fig. 72) in the hydrogen
atom as well as binding energies of the first electron
(Fig. 72) in the hydrogen
atom as well as binding energies of the first electron ![]() of the oxygen atom
with its nucleus have the values, which are close in their magnitude on the
corresponding energy levels (Table 35, 36) and Fig. 72.
of the oxygen atom
with its nucleus have the values, which are close in their magnitude on the
corresponding energy levels (Table 35, 36) and Fig. 72.
The new theory puts the following questing
before us: how many electrons are in water molecule? Do the first and the
second electrons of oxygen atom always remain in their cells when the electrons
of hydrogen atoms come nearer to them? We have no definite answer to this
question and we suppose that all possible variants are realized. In some cases
the first and the second electrons of oxygen atom are absent in water molecule,
and their places are occupied by the electrons of hydrogen atoms. But the
presence of these electrons in water molecule is not excluded, because when
valence electrons of the atoms unite,
they are connected not only with the protons of the neighboring atom, but also
with its valence electrons. Taking into consideration the above-mentioned
facts, the structure of water molecule can differ in quantity of electrons in
it, and it is necessary to give a name to these structures.
We have called the structure of water molecule with a
complete set of electrons the first
model (Fig. 72). There exist the possibilities of the formation of
water molecules not with ten electrons, but with eight electrons (Fig. 73). Let us call such model the second one.
The main
differences between the first (Fig. 72) and the second (Fig, 73) models of
water molecule are in the fact that two coupled electrons are in the cells of
the first electron and the second (axial) one of the oxygen atom of the first
model of water molecule; in the second model of water molecule, one electron is
situated in these cells, and we have every reason to call them non-coupled
electrons (Fig. 73).
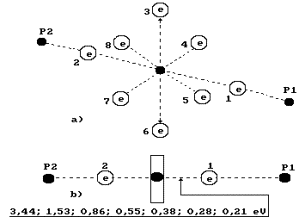
Fig. 73. Diagram
of the second (discharged) model of
water molecule.
When coupled
electrons are arranged only at one end of the oxygen atom axis (to the right),
we’ll call such model the third one (Fig. 74).
If the hypothesis
concerning different quantity of electrons in water molecules is confirmed,
this fact will be a decisive one in obtaining surplus energy during water
electrolysis. It will determine the reason of positive and negative results of
various experiments, which have been carried our for the check of the fact of
existence of additional energy during water electrolysis [67]. If water
contains more charged molecules, the experiment will give a positive result.
When there are many discharged molecules, the result will be negative. The
approximate calculations demonstrate availability of a difference in mass of
one liter of charged and discharged water. It can be registered with the help
of modern measurement devices.
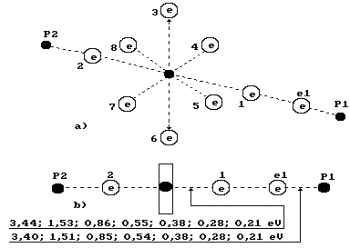
Fig. 74.
Diagram of the third model of water molecule.
Later on we’ll show
that the water molecule clusters, which have positive and negative charges, are
formed before the thunderstorm discharges in the clouds. Different temperature
in the clouds is the reason of the division of the water molecule clusters. Now
we have an opportunity to calculate
this difference and to try to model the thunderstorm discharge process and to
make it a controlled one.
It is known that
water can have alkali or acid properties. Alkali properties are formed at the
expense of the increased content of hydroxyl ![]() in water (Fig. 75).
in water (Fig. 75).
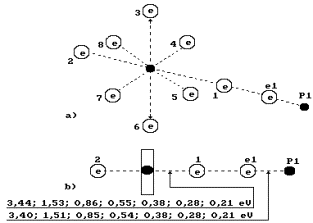
Fig. 75. Diagram of structure
of hydroxyl ![]()
As it is considered
now, acid properties of water are formed by free protons ![]() , but we do not agree with this idea, because the proton is a
very active formation, that’s why it cannot exist in water in a free state.
Acid properties of water are formed by an increased content of positively charged ions of hydroxonium
, but we do not agree with this idea, because the proton is a
very active formation, that’s why it cannot exist in water in a free state.
Acid properties of water are formed by an increased content of positively charged ions of hydroxonium ![]() (Fig. 76).
(Fig. 76).
In all models of
water molecules (Fig. 72-74) the third – eighth electrons of oxygen atom remains free forming a negative
potential zone on its surface. The values of the third and the fourth
potentials of ionization of the oxygen atom point out to the fact that the ring
electrons are arranged nearer to the nucleus of the oxygen atom than the axial
ones, that’s why the majority of their electrical and magnetic lines of force
is included in the bond with the nucleus of the oxygen atom, that’s why they
are less a active than the first electron and the second one. One of the ring
electrons should be lifted in its cells and be removed from the nucleus of the
oxygen atom in order to be connected with the proton or the electron of the
neighboring atom.
In order to realize such process it should
absorb the proton of the environment.
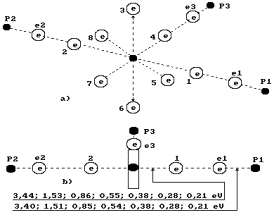
Fig. 76. Structure
of the ion of hydroxonium ![]()
If it takes place,
it will move off the nucleus, come nearer to the surface of the atom, and the
conditions will appear for the connection of the lines of force of its magnetic
field with the lines of force of magnetic field of the proton or the electron.
If one of circular electron of oxygen atom unites with the proton, the ion
of hydroxonium ![]() is formed,
which forms acid properties of water (Fig. 76). If the events develop in such a
way, three zones with positive potential are formed on the surface of water
molecule, and it becomes a positively charged ion
is formed,
which forms acid properties of water (Fig. 76). If the events develop in such a
way, three zones with positive potential are formed on the surface of water
molecule, and it becomes a positively charged ion ![]() , which is called hydroxonium (Fig. 76). As we have already
proved that there are no protons in free state in electrolytic solution, it
means that acid properties of the solution are determined not by the proton
(positive ion
, which is called hydroxonium (Fig. 76). As we have already
proved that there are no protons in free state in electrolytic solution, it
means that acid properties of the solution are determined not by the proton
(positive ion ![]() ), but by the positive ion of hydroxonium
), but by the positive ion of hydroxonium ![]() . We know that the process of the removal of the electron
from the atomic nucleus is accompanied by the absorption of the photons form
the environment. That’s why hydroxonium ion formation process will be an
endothermic one.
. We know that the process of the removal of the electron
from the atomic nucleus is accompanied by the absorption of the photons form
the environment. That’s why hydroxonium ion formation process will be an
endothermic one.
Hydrogen peroxide H2O2
is formed from water as well. There are two oxygen atoms 2O and two hydrogen
atoms 2H in its structure (Fig. 77).
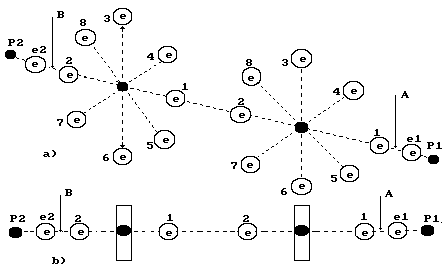
Fig. 77. Diagram of
hydrogen peroxide model H2O2
Binding energies
between the protons and the electrons taken from the calculation results of the
spectra of the atoms and the ions are given in the diagrams of water molecules
(Figs 72-74), hydroxyl (Fig. 72) and hydroxonium (Fig. 73). In our previous
publications [70] we have treated with faith the calculation of binding
energies between the atoms in the molecules carried out by chemists, that’s why
we have taken a part of the values of these energies from the chemical
calculations and a part from the spectrum calculation results. But we have
already shown that binding energies of the electrons with atomic nuclei
determined not with the help of chemical calculations, but from the results of
spectroscopy of the atoms and the ions are closer to the data obtained during
water electrolysis. That’s why we’ll use mainly these data (Figs 72-76).
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index