<<
Back to Kanarev's Physchemistry Book Index
10.11.
Structure of the Water Molecule
Water is the most
widely spread chemical combination. Numerous works are devoted to the study of
this combination. In 1951, the Danish scientist N. Bjerrum studied the
structure and the properties of ice and suggested the water molecule model
given in Fig. 63 [46], [58], [63].

Fig. 63. Diagram of
the water molecule structure
In this diagram,
one can see the impression of the notions concerning the orbital movement of
the electron in the atoms. Having determined the lack of orbital movement of
the electrons in the atoms we did not dare changing the arrangement of the
water molecule and gave it in our previous publications in the way, which is
given in Fig. 64.
We cannot help
admiring the exactness, with which valence angle 104.5° is determined between the
hydrogen atoms in water molecule. It is calculated wit the help of indirect
methods, which originate from the notions concerning the distribution of the
electrons along the orbitals. These notions have been formed on the basis of
the solutions of Schroedinger’s equations, which, as it is known, predict only
density of probability of stay of the electron in this or that area of the
atom.
We have already
shown that Schroedinger’s equation operates outside the framework of the
space-matter-time unity axiom, and due to this fact it distorts the actual
physical and chemical phenomena of the microworld. That’s why we have every
reason to doubt in the structure of the water molecule suggested by Bjerrum.
The diagram of the
water molecule model shown in Fig. 64 takes into account the existing notions
concerning the structure of this molecule, but without the orbital movement of
the electrons. We have used
successfully this model for analysis of physical and chemical processes during
plasma electrolysis of water. Now the model of the nucleus of the oxygen atom
determined by us makes us change the existing notions concerning the water
molecule structure. Showing a new structure of the water molecule we’ll note
that it will not only change our energy calculations for plasma water
electrolysis as we show later on, but allows us to advance in our search and to
describe the processes, which take place in the thunderstorms discharges in the
clouds.
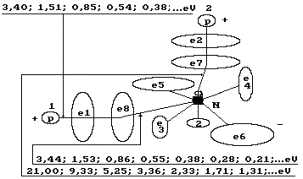
Fig. 64. Diagram of
the water molecule model: 1,2,3,4,5,6,7,8 are the numbers of the electrons of
the oxygen atom, N is the nucleus of the oxygen atom, P is the nuclei of the
hydrogen atoms (the protons); ![]() and
and ![]() are the numbers of
the electrons of the hydrogen atoms.
are the numbers of
the electrons of the hydrogen atoms.
In Fig. 65, the
water molecule structure is shown, which originates from the structures of the
atomic nuclei of oxygen and hydrogen. Two electrons 1 and 2 of the oxygen atom
are arranged on the atomic axis, and six rest ones are arranged in a circle,
which is perpendicular to the axis. One can suppose that the total
electrostatic field of six electrons arranged in a circle (let us call them
circle electrons) remove the first and the second axis electrons at a larger distance than the distance from
the atomic nucleus, at which he circle electron are situated. That’ why the
axis electrons of the oxygen atom are the main valence electrons of his atom.
The electrons of the hydrogen atom are connected to them, and a water molecule
is formed (Fig. 65).
The electrons of
the hydrogen atom are designated with the symbols ![]() and
and ![]() , and the protons of the hydrogen atoms are designated with
the symbols
, and the protons of the hydrogen atoms are designated with
the symbols ![]() and
and ![]() . Let us remind that we give the numbers to the electrons in
accordance with the sequence of the increase of their ionization potentials. We
have given the first number to the electron of the oxygen atom, which has the
least ionization potential
. Let us remind that we give the numbers to the electrons in
accordance with the sequence of the increase of their ionization potentials. We
have given the first number to the electron of the oxygen atom, which has the
least ionization potential ![]() =13.618 eV. We have given the second number to the second
electron of the oxygen atom, which has ionization potential of
=13.618 eV. We have given the second number to the second
electron of the oxygen atom, which has ionization potential of ![]() =35.116 eV.
=35.116 eV.
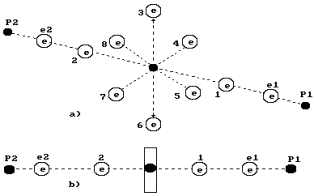
Fig. 65. Diagram of
the water molecule model: spatial diagram; b) linear diagram
It should be noted
that when ambient temperature is decreased, the circle electrons of the oxygen
atom in the water molecule come close to the nucleus (the axis of the water
molecule, and with their total electrical field they remove two main axis
electrons from the nucleus, which form connections in the water molecule
clusters. Due to it, when water is frozen, the connections between the
molecules in the clusters become longer, and frozen water molecule becomes
longer and increases the volume of the clusters. This is the main reason of
expansion of water when it is frozen.
10.12. Structure of
the Ammonia Molecule
Ammonia NH3
is colourless gas with pungent smell. It is clear from Fig. 66 that one
hydrogen atom (the electron ![]() and the proton
and the proton ![]() ) with its electron becomes connected with the axis electron
of the nitrogen atom. Two other hydrogen atoms are connected by their electrons
with two electrons of the nitrogen atom arranged in its ring.
) with its electron becomes connected with the axis electron
of the nitrogen atom. Two other hydrogen atoms are connected by their electrons
with two electrons of the nitrogen atom arranged in its ring.
A question arises
at once: why are the hydrogen atoms connected by their electrons with the
electrons of the nitrogen atom? The structures of both the electron and the
proton are such that the protons of the hydrogen atoms can be connected with
the electrons of the nitrogen atom. We have no answer to this question.
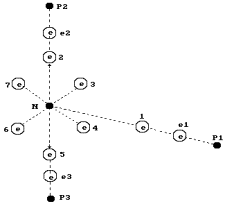
Fig. 66. Diagram of
the ammonia molecule ![]() : N is the nucleus of the nitrogen atom; 1,2,3,4,5,6 and 7
are the electrons of the nitrogen atom,
: N is the nucleus of the nitrogen atom; 1,2,3,4,5,6 and 7
are the electrons of the nitrogen atom, ![]() ,
, ![]() and
and ![]() are the electrons of
three hydrogen atoms;
are the electrons of
three hydrogen atoms; ![]() ,
, ![]() are the protons of
the hydrogen atoms.
are the protons of
the hydrogen atoms.
We think that the
above mentioned method of formation of the structures of the atoms and the
molecules is enough in order to build the models of other atoms and molecules.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index