<<
Back to Kanarev's Physchemistry Book Index
10.13. Energy Balance of Fusion Processes of Molecules of Oxygen, Hydrogen and Water
In engineering
practice connected with ventilation system servicing, a phenomenon of excessive
thermal energy in circulating air has been found. Similar phenomenon has been
registered in water circulation systems with the devices for its active
cavitations. The results of our investigations explain not only a cause of
these phenomena, but they give an opportunity to perform quantitative
calculations of energy processes, which generate additional thermal energy.
The atom of oxygen
is the eighth element of the periodical table. The structure of this atom and its nucleus is shown at Fig. 61.
The least ionization energy of
the electron of the oxygen atom is equal to ![]() =13.618 eV. Binding energy of this electron with the atomic
nucleus corresponding to the first energy level is equal to
=13.618 eV. Binding energy of this electron with the atomic
nucleus corresponding to the first energy level is equal to ![]() =13.752 eV. The other binding energy of this electron is in
Table 21.
=13.752 eV. The other binding energy of this electron is in
Table 21.
It is known that
the fusion process of the oxygen molecules is accompanied with a release of 495
kJ/mole of energy, or in calculation for one molecule
![]() (255)
(255)
What principle does
the nature go by distributing energy 5.13 eV between the oxygen molecule
electrons (Fig. 67, a)? Energy of 5.13
eV is a thermal binding energy between the electrons 1 and 2’ of two oxygen
atoms (Fig. 67, a). When the oxygen molecule is formed, it is emitted in the
form of the photons by the electrons, which enter the bond. It appears from
this that it is equal to an amount of energies of two photons emitted by these
electrons. Consequently, each contacting electron emits a photon with energies of 5.13/2=2.565 eV (Fig.
67). According to Table 20, in this case the valence electrons are situated
between the second energy level and the third one.
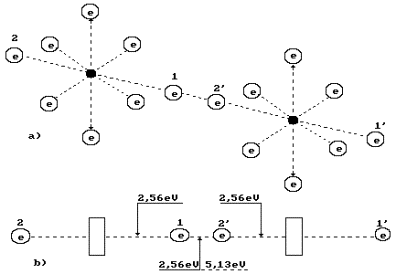
Fig. 67. Diagram of
binding energy distribution between the electrons in the oxygen molecule
Two oxygen atoms
are connected into a molecule in an excitation state. The excitation state is
the state of an atom when its valence electrons are situated at such distances
from the nuclei when the binding energy between them is reduced to the
thousandth fractions of an electron-volt. In such state, the atom can loose an
electron and become an ion. Otherwise, without loosing electrons it is
connected with an electron of the neighbouring atom by the valence electron,
and a process of oxygen molecule formation begins. It is an exothermic process
when the axis valence electrons 1 and 2’ emit the photons, descend on lower
energy levels and release 2.565x2=5.13 eV. Let us pay attention to the fact
that energy 5.13 eV is released by two electrons, which form a bond with energy
of 2.56 eV. In modern chemistry, this bond is called a covalent bond. In order
to break this bond, it is necessary to use 2.56 eV of mechanical energy. For
thermal cleavage of this bond, double quantity of energy is required, i.e. 5.13
eV. It is explained by the fact that the photon energy of 5.13 eV is absorbed
by two electrons simultaneously. Only in this case, both electrons will be
transferred to the highest energy levels with minimal binding energy when they
are disconnected, and each oxygen atom becomes a free one.
Thus, energy
expenses for an oxygen molecule destruction depend on an impact method on the
bond. During thermal impact on the bond it is destroyed when energy is 5.13 eV.
During mechanical impact of the bond, it is necessary to spend 2.56 eV of
energy in order to destroy this bond. It appears from this that energetic of
fusion process of the oxygen molecule depends on its destruction method.
After the thermal
destruction of the oxygen molecule its formation process begins from emission
of the photons with energies of 2.56 eV by both valence electrons, and the
previous electrodynamics binding energy (2.56 eV) is restored between the
electrons of both atoms.
Thus, during the
thermal destruction of the oxygen molecule the same amount of thermal energy is
spent, which is released during its further formation. No additional energy
appears during thermal dissociation of the oxygen molecule and its further
fusion.
If the oxygen
molecule is destroyed by a mechanical method, it is necessary to spend 2.56 eV
of mechanical energy for this purpose. Valence electrons of the oxygen atoms
are in a free state by a lack of energy corresponding to such state as there is
no absorption process of 2.56 eV of energy by each of them. The electrons
cannot remain in such state, they should replenish immediately the energy,
which they have failed to receive during a mechanical break of the bond between
them. Where should they take it? There is only one source: environment, i.e.
physical vacuum filled with ether. They convert ether into energy of 2.56 eV
immediately.
The next stage is a connection of two oxygen atoms, which
valence electrons have replenished the reserves of their energy at the expense
of ether. This process is accompanied by emission of the photons with energies
of 2.56 eV by two electrons. Thus, energy of absorbed ether is converted into
thermal energy of the photons. If we spend 2.56 eV of mechanical energy for the
oxygen molecule destruction, we’ll get double quantity of energy (2.56x2=5.13)
eV during further fusion of this molecule. Additional energy is equal to 2.56
eV.
Many experimental
data show that in ventilation systems thermal energy of circulating air exceeds
electric energy spent for a fan drive. Now we know that this energy is
generated at mechanical failure of covalent bonds in the molecules of the
gases, which the air consists of.
Using the
above-mentioned method, we’ll analyse water molecule energetic, which sometimes
generates additional thermal energy. A water molecule consists of one oxygen
atom and two hydrogen atoms. Binding energies ![]() of the hydrogen atoms
with its nucleus are given in Table 4.
of the hydrogen atoms
with its nucleus are given in Table 4.
It is known that a connection of hydrogen with oxygen is
accompanied by an explosion, but its cause remains unknown. Let us try to find
it.
Hydrogen molecule
fusion energy is equal to 436 kJ/mole, or 4.53 eV per molecule. As the molecule
consists of two atoms, the above-mentioned energy is distributed between them.
Thus, energy of one bond between the hydrogen atoms is equal to 2.26 eV (Fig.
68). At thermal failure of this bond, double quantity is required 2.26x2=4.53
eV.
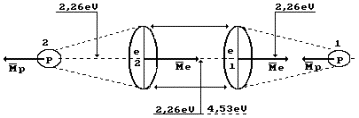
Fig. 68. Hydrogen
molecule
In order to form
two water molecules, it is necessary to break two hydrogen molecules and one
oxygen molecule into atoms. If the destruction processes of the above-mentioned
molecules are carried out with a thermal method, 4.53+4.53=9.06 eV are required
for the destruction of two hydrogen molecules, and 5.13 eV are required for the
destruction of one oxygen molecule. Totally, it is 14.19 eV.
It is known that
during fusion of one mole of water 285.8 kJ or ![]() per molecule are released. As a
water molecule consists of one oxygen atom and two hydrogen atoms, 2.96/2=1.48
eV falls per bond (Fig. 69). It appears from this that the electrons of the
atoms of hydrogen and oxygen in the water molecule are at the usual temperature
1.48/2=0.74 eV between the forth energy level and the fifth one (Table 4 and
21).
per molecule are released. As a
water molecule consists of one oxygen atom and two hydrogen atoms, 2.96/2=1.48
eV falls per bond (Fig. 69). It appears from this that the electrons of the
atoms of hydrogen and oxygen in the water molecule are at the usual temperature
1.48/2=0.74 eV between the forth energy level and the fifth one (Table 4 and
21).
Thus, when two hydrogen molecules and
one oxygen molecule are destroyed by the thermal method, 14.19 eV are spent. As
a result of fusion of two water molecules, 2.96x2=5.98 eV are released. It
conflicts with the fact that water molecule fusion process is an exothermic one
with a release of 2.96 eV by one molecule. The given calculation shows that
(14.19-5.98)/2=4.10 eV are absorbed during fusion of one water molecule. What
is the cause of this contradiction?
The oxygen atom in
the water molecule should reduce its volume when the transition from gaseous
state into liquid state takes place. It will happen when the rings electrons of
the oxygen atom descend on lower energy levels (nearer to the nucleus). They
will emit the photons, and we know their total energy. It is equal to energy
spent to destruction of two hydrogen molecules and one oxygen molecule, i.e.
14.19 eV. As two water molecules have 12 ring electrons, each of them will emit
14.19/12=1.18 eV (Fig. 69). It is more than axis electron binding energy (0.74
eV) with the nucleus, and it shows that the ring electrons are situated nearer
to the nucleus than the axis ones.
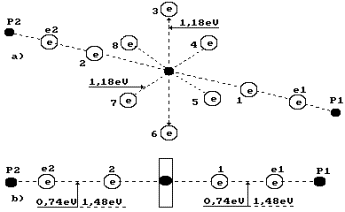
Fig. 69. Diagram of
water molecule:
1,2,3,4,5,6,7,8 are
the numbers of the electrons of the oxygen atom; P1, P2
are the nuclei of the hydrogen atoms (the protons); e1 and e2 are the numbers
of the electrons of the hydrogen atoms
In this case,
quantity of the energy produced due to fusion of two water molecules
(14.19+5.98) eV exceeds the energy spent for the destruction of two hydrogen
molecules (9.06 eV) and one oxygen molecule (5.13 eV). The formed energy
difference of 5.98 eV is divided between two water molecules. It means that
5.98/2=2.99 eV or 285.8 kJ/mole fall per molecule. It corresponds to the
existing experimental data completely.
The above-mentioned
facts clarify a cause of the explosion, which takes place when hydrogen is
combined with oxygen. Simultaneous transition of six ring electrons of each
oxygen atom in the nascent water molecules to lower energy levels is
accompanied by simultaneous emission of the photons, which generate explosion
phenomenon.
Let us pay
attention to the fact that two binding energies between valence electrons e2
and 2 and between 1 and e1 are shown in Fig. 69, b. Energy of one
electrodynamics bond is equal to 0.74 eV. If this bond is destroyed by the
thermal method, 0.74x2=1.48 eV is required. This energy will be released during
further fusion of the water molecule from hydrogen atom ![]() and hydroxyl ion
and hydroxyl ion ![]() . In this case, no additional energy is generated.
. In this case, no additional energy is generated.
It appears from
this that the given bond is destroyed by the mechanical method spending 0.74 eV
per bond, each electron will have energy deficit equal to 0.74 eV after bond
failure. This energy will be absorbed from the environment immediately and will
be emitted during the repeated fusion of the water molecule from the hydrogen
atom ![]() and the hydroxyl ion
and the hydroxyl ion ![]() . At mechanical failure of one bond of water molecule, the
covalent chemical bond forms 0.74 eV of additional thermal energy, which is
registered in the water cavitation systems constantly (as we have already
noted).
. At mechanical failure of one bond of water molecule, the
covalent chemical bond forms 0.74 eV of additional thermal energy, which is
registered in the water cavitation systems constantly (as we have already
noted).
It is known that
the water molecules unite and form clusters. If the bonds between the molecules
in the clusters are covalent ones, mechanical destruction of these bonds should
be accompanied by a release of additional thermal energy as well.
In the Russian
market, three firms (Yusmar, Termovikhr and Noteka) sell cavitation water
heating equipment with energy efficiency index of 150%. Soon, an air heating
device with the same efficiency will be produced. The processes of mechanical
destruction of covalent bonds of the air gas molecules, molecules and clusters
of water and their further fusion serve as a source of additional energy
generated by these devices.
Physical vacuum serves as a source of additional energy
generated by these heating elements. The electrons of the clusters and
molecules forming covalent bonds extract this energy from physical vacuum after
mechanical destruction of these bonds and release it during further fusion of
the molecules and the clusters.
10.14.
Energetics of Chemical Bonds of the Ozone Molecule
Ozone is a gaseous substance, which consists of
three-atom molecules ![]() . In
order to destroy the oxygen molecule, it is necessary to spend 5.13 eV of energy.
During fusion of two ozone molecule, 2.99 eV of energy are released. As a
result, energy difference
5.13-2.99=2.15 eV takes place.
The authors of the fundamental monograph [195] devoted to ozone assert that energy
of 2.15 eV is absorbed by the third unknown particle
. In
order to destroy the oxygen molecule, it is necessary to spend 5.13 eV of energy.
During fusion of two ozone molecule, 2.99 eV of energy are released. As a
result, energy difference
5.13-2.99=2.15 eV takes place.
The authors of the fundamental monograph [195] devoted to ozone assert that energy
of 2.15 eV is absorbed by the third unknown particle ![]() , which takes part in this process. The oxygen atom, the
molecules of oxygen and ozone as well as any other molecule being present in
the ozone molecule fusion zone can play the role of this particle. Such
assumption is made for the purpose that the law of conservation of energy will
not be violated. The ozone molecule fusion reaction is written in such a way
, which takes part in this process. The oxygen atom, the
molecules of oxygen and ozone as well as any other molecule being present in
the ozone molecule fusion zone can play the role of this particle. Such
assumption is made for the purpose that the law of conservation of energy will
not be violated. The ozone molecule fusion reaction is written in such a way
![]() .
.
At any rate, it is a strange assumption.
It is known that each portion of energy has its owner in the processes of
fusion and dissociation of the molecules. That’s why it is necessary to find a
true owner of energy of 2.15 eV [195].
Prior to the
analysis of energetics of the chemical bonds of the ozone molecule, it is necessary
to understand energetics of the chemical bonds of the atom ![]() and the oxygen molecule
and the oxygen molecule ![]() .
.
The oxygen molecule structure
is given in Fig. 67, a. It is formed by means of a connection of unlike magnetic
poles of axis electrons of two oxygen atoms [196]. As it is clear, the oxygen
molecule has fourteen free electrons, which are ready for bond. The axial
electrons 1’ and 2 are the most remote ones from the structure of the whole molecule;
they have the greatest activity, i.e. aptitude for bond with the electrons of
other atoms [196].
It is known that
the fusion process of the oxygen molecules is accompanied with a release of 495
kJ/mole of energy, or in calculation for one molecule
![]() (4)
(4)
What principle does
the nature go by distributing energy 5.13 eV between the oxygen molecule
electrons (Fig. 67, b)? Energy of 5.13
eV is a thermal binding energy between the electrons 1 and 2’ of two oxygen
atoms (Fig. 67, a). When the oxygen molecule is formed, it is emitted in the
form of the photons by the electrons, which enter the bond. It appears from
this that it is equal to an amount of energies of two photons emitted by these
electrons. Consequently, each contacting electron emits a photon with energies
of 5.13/2=2.565 eV (Fig. 67). According to Table 21, in this case the valence
electrons are situated between the second energy level and the third one [196].
Two oxygen atoms
are connected into a molecule in an excitation state. The excitation state is
the state of an atom when its valence electrons are situated at such distances
from the nuclei when the binding energy between them is reduced to the
thousandth fractions of an electron-volt. In such state, the atom can loose an
electron and become an ion. Otherwise, without loosing electrons it is
connected with an electron of the neighbouring atom by the valence electron,
and a process of oxygen molecule formation begins. It is an exothermic process
when the axis valence electrons 1 and 2’ emit the photons, descend on lower
energy levels and release 2.565x2=5.13 eV.
In order to destroy
the oxygen molecule and to form the ozone molecule, spark discharge or photon
flux with energies, which are somewhat larger than binding energy of
2.565х2=5.13 eV between the oxygen atoms in its molecule, are used (Fig.
67, b). It is known that ozone is formed during ultraviolet radiation with the
wavelength of ![]() . Photon energy, which corresponds to this wave
length, is equal to
. Photon energy, which corresponds to this wave
length, is equal to
![]()
As ozone is formed
according to equation![]() , it is necessary to
destroy one oxygen molecule
, it is necessary to
destroy one oxygen molecule ![]() for fusion of two
molecules of ozone
for fusion of two
molecules of ozone ![]() . To this effect, it is necessary to excite 2 electrons having
spent 2.565x2=5.13 eV for this purpose (Fig. 67).
. To this effect, it is necessary to excite 2 electrons having
spent 2.565x2=5.13 eV for this purpose (Fig. 67).
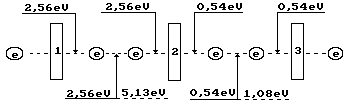
Fig, 70. Diagram of binding
energy distribution in the ozone molecule ![]()
It is known that 144 kJ
are released during dissociation one mole of ozone. As a result, we have per
molecule:
![]() (256)
(256)
Ozone formation process begins when even the smallest
temperature reduction takes place in ozone where the oxygen atoms are in
excited state. Their valence electrons are connected with the valence electrons
of the oxygen atoms in its molecules and emit the photons with such total
energy that the remainder of energy absorbed earlier (5.13 eV) will be equal to
endothermic energy of 1.49x2=2.99 eV of formation of two ozone molecule. Energy
of the emitted photons will be equal to 5.13-2.99=2.15 eV. This energy is spent
for formation of the bonds in two ozone molecules, which have 4 valence
electrons. Binding energy corresponding to one electron is equal to 2.15/4=0.54
eV (Fig. 70). In this case, valence electrons are almost on the fifth energy
levels (Table 21).
As it is clear (Fig. 70), the ozone molecule is longer
than the oxygen molecule (Fig. 67); binding energy (0.54 eV) between the third
one, which is connected by the oxygen atom, is fivefold less than between the
oxygen atoms (2.56 eV) in its molecule. As a result, stability of the ozone
molecule is less than stability of the oxygen molecule, and it is destroyed
easier forming the oxygen molecules and its atoms. For this effect,
availability of light photons, which energy is changed within the range of
0.016-3.27 eV, is enough (Table 34).
Table 34. Electromagnetic
emission scale ranges
|
Bands |
Wave-length, m |
Energy, eV |
|
1. Low- frequency band |
|
|
|
2. Broadcast band |
|
|
|
3. Microwave band |
|
|
|
4. Relic band (maximum) |
|
|
|
5. Infrared band |
|
|
|
6. Light band |
|
|
|
7. Ultraviolet band |
|
|
|
8. Roentgen band |
|
|
|
9. Gamma band |
|
|
After destruction of two ozone molecules, the valence
electrons of the separated oxygen atoms pass into exited state absorbing
0.54x4=2,15 eV of energy. When they reach the highest energy levels, they are separated;
after the free state stage, they form an oxygen molecule emitting the photons
with total energy equal to 5.13 eV. Difference between emitted energy of 5.13
eV and energy of 2.15 eV absorbed by four electrons is equal to the
dissociation energy of two ozone
molecule of 2.99 eV, or 1.44 kJ/mole.
The disclosed models of the oxygen atom and molecule as
well as empirical mathematical law of formation of the spectra of the atoms and
the ions give the possibility to make a detailed analysis of energy balance of
formation of the molecules of oxygen and ozone, which corresponds to the experimental
data completely.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index