<<
Back to Kanarev's Physchemistry Book Index
9.8. Diagram of Nucleus of Boron Atom
Boron is the fifth element in the
periodic table of chemical elements. It seems that the majority of the atoms of
this element should have the nuclei with five protons and five neutrons, but it
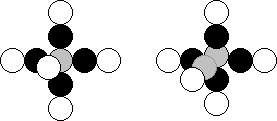
is not the case. Only 20% of the boron atoms have the nuclei with five protons and
five neutrons (Fig. 25, a), and 80% of atoms of this element have the nuclei,
which consists of five protons and six neutrons (Fig. 25, b) [120], [121].
(Axial neutrons are shown in grey).
a) b)
Fig. 25. Diagrams of the nuclei of the
boron atom: a) with five neutron, b) with six neutrons
(the protons are shown in white, the neutrons are shown in black)
In the diagram (Fig. 25,
a), the central neutron is shown in grey; in the diagram of (Fig. 25, b), two
neutrons forming the axis of the nucleus are shown in grey [120], [121].
The analysis of the diagrams of the
composition of the boron atom (Fig. 25, a and b) proves that an additional
neutron (Fig. 25, b) separates the fifth proton from the rest four protons at a
large distance. Due to this fact, in the nucleus, which diagram is shown in
Fig. 25, b, the electrostatic forces of repulsion of the fifth proton from the
rest four protons are less than in the nucleus shown in Fig. 25, a. Thus, the
additional neutron improves strength of the nucleus of the boron atom, that’s
why there are more nuclei of the boron atom with six neutron than with five
neutrons in Nature.
Let us pay attention to a number of contacts
of the central neutron with the rest neutrons. There are five of them and one
free. If each contact corresponds to a definite magnetic pole of the magnetic
field of the neutron, the total number of contacts should be even, i.e. it
should be six. One contact (it means one magnetic pole) of the central neutron
is free. Later on we’ll see that it is occupied in the structure of the nucleus
of the carbon atom when a diamond is formed from it [109], [121].
Thus, we get additional confirmations of
connection of the protons with the neutrons in nuclei of the atoms by means of
unlike magnetic poles only, not nuclear forces. Each neutron has a compound magnetic field, which helps to
generate six magnetic poles: three north poles and three south poles.
9.9.
Diagrams of Nucleus of Carbon Atom
Carbon is supposed to be a base of life, because it forms a
large number of bonds with the atoms of other chemical elements. Let us consider
a cause of its such activity.
A flat nucleus of this element is shown in Fig. 26, a. Involuntarily, one recollects scaly flat composition
of graphite, which is formed from carbon. Such substance is formed from the
carbon atoms, which nuclei have flat structure made of six protons and six
neutrons. But there is carbon with
other, spatial arrangement of the nucleus in Nature. Mechanical properties of
diamond (Fig. 26, b), which consists of carbon as well, differ from mechanical
properties of graphite significantly [109], [121].
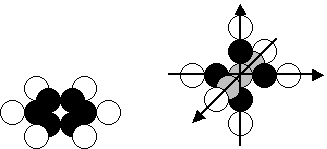
a) b)
Fig. 26. Structural diagrams of carbon atom: diagram of flat nucleus; b) diagram of spatial nucleus
Now we can see that the form of the
carbon nucleus determines properties of a substance, which consists of the atoms
of this chemical element.
The
structure of other nucleus of the carbon atom is shown in Fig. 26, b. This
structure has 7 neutrons. One of them is situated in the centre of the spatial
coordinate system, and tree pairs of other neutrons are directed along three
coordinate axes. A proton is connected to each outer neutron along these axes.
Thus, spatial nucleus of the carbon atom is an ideal point of the lattice. Such
structure of the nucleus provides strength of the diamond crystals.
Experimental nuclear spectroscopy proves that 98.90% of the carbon nuclei
contain 6 protons and 6 neutrons, and only 1.10% of the nuclei of this element
has a surplus neutron. Now we see that they are the diamond atoms (Fig. 26, b).
Let us pay attention to limit symmetry
of both nuclei of the carbon atom. Flat symmetric nucleus belongs to carbon,
which forms organic substances.
It is clear from the second structural
diagram (Fig, 26, b) of the nucleus of the carbon atom that the neutron has a
compound magnetic field, which consist of six magnetic poles. In all cases
being considered by us, magnetic pole of the proton remains similar to magnetic
pole of the bar cylindrical magnet.
9.10. Structure of Nucleus of Nitrogen Atom
Nitrogen is the seventh chemical element
in the periodic table. There are 99.63% of the nitrogen atoms in Nature, which
nuclei consist of 7 neutrons and 7 protons (Fig. 27, a). The eighth surplus
neutron is available in 0.37% of the nuclei of the atoms of this element [120],
[121].
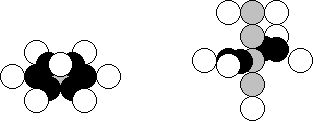
a) b)
Fig. 27. Diagram of the nucleus of the nitrogen atom
Six neutrons arranged in one plane
have six free magnetic poles directed to the centre of circumference, which
they form (Fig. 27, a). As each neutron has four magnetic poles in one plane,
the seventh neutron occupies free space in the centre, and the seventh proton
is connected to it from above (Fig. 27, a). As the central neutron has one free
magnetic pole in its lower part, the eighth neutron can be connected to it
forming a nucleus of the nitrogen isotope. It is obvious that other neutrons can be connected to the
neutron increasing the number of the isotopes of this element. The nuclei of
the isotopes of the nitrogen atom can have four surplus neutrons [120], [121].
It should be noted that it is impossible to form the nucleus of
the nitrogen atom from the spatial structure of the nucleus of the carbon atom
(Fig. 26, b). This structure has already had 7 neutrons and 6 protons, there is
no place for the seventh proton. But if
one surplus neutron is added to the spatial structure of the nucleus of
the carbon atom, the conditions take place for the formation of a nucleus of
the nitrogen atom with seven protons and eight neutrons (Fig. 27, b).
As there are only 0.37% of the nuclei
of the nitrogen atoms with eight neutrons, we have every reason to believe that
the majority of the nuclei of the nitrogen atom should have a flat nucleus of
the nitrogen atom (Fig. 27,a) [120],
[121].
9. 11. Structure of Nucleus of Oxygen Atom
The experimenters attribute magical
properties of stability to the nucleus of this atom. The number, which corresponds
to the ordinal number of this element, is also considered to be a magic one.
Symmetry of arrangement of the neutrons and the protons in this nucleus proves
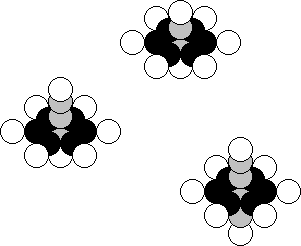
this fact (Fig. 28, a).
a)
b)
c)
Fig. 28. Diagram of the nucleus of the
oxygen atom
The nucleus of this atom has 8 protons and 8 neutrons. In the
central part of the nucleus, two neutrons are arranged along its axis, and two
protons are connected to them. As a result, an ideal symmetrical, i.e. stable
structure is formed. As the nucleus of the oxygen atom has symmetrical spatial
structure, the possibilities of chemical activity are increased greatly.
In Nature, 99.762% of the oxygen
atoms have eight neutrons and eight protons (Fig. 28, a). Analysis of the diagram
of a symmetrical nucleus of the oxygen
atom shows that the neutron can penetrate between the upper and the power
central protons, and the nuclei of the oxygen isotopes are formed. There are
0.038% of the nuclei of the oxygen atom with one surplus neutron (Fig. 28, b)
and 0.200% of the nuclei with two surplus neutrons in Nature (Fig. 27, c). The
nucleus of the oxygen atom can have five surplus neutrons [120], [121].
It is necessary to note impossibility of formation of the
spatial structure of the nucleus of the oxygen atom. In Fig. 26, b, where the
spatial nucleus of the nitrogen atom with eight neutrons and seven protons is
shown, there is no place for the eighth proton of the nucleus of the oxygen
atom.
9.12.
Diagram of Nucleus of Fluorine Atom
Fluorine is the ninth element of the
periodic table. It is situated in the seventh period of this table. Its stable
nucleus has 9 protons and 10 neutrons (Fig. 29). When the nucleus of this
element is formed, two neutrons and two protons are connected to one of the
protons of the nucleus of the oxygen atom arranged along the axis of the
nucleus.
As fluorine is situated in the same group with hydrogen in
the periodic table, its nucleus should have the elements of the nucleus of this
atom (Fig. 21, b). The protons arranged at the ends of the axis of the nucleus,
play the role of such element [120], [121].
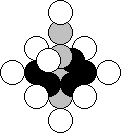
Fig. 29. Diagram of the nucleus of the fluorine atom
9.13. Diagram of
Nucleus of Neone Atom
Neon is the tenth element of the
periodic table. It is situated in the eight group of this table that is why it must contents
the elements of the nucleus of helium atom.
There are 90.51% of the nuclei of this
atom with 10 protons and 10 neutrons in Nature (Fig. 30, a). In fact, 0.27% of
the nuclei of this element have one surplus neutron (Fig. 30, b) and 9.22% of
the nuclei have two neutrons (Fig. 30 c,).
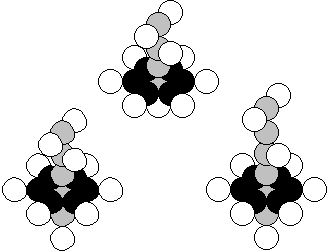
a)
b) c)
Fig. 30. Diagram of the nucleus of the neon atom
In order to preserve symmetry of the nucleus,
it is built by means of connection of one neutron and one proton to the axial
chain of the nucleus of the fluorine atom. We get a symmetrical structure (Fig. 30, a).
If one neutron is added to the lower part of the axis of
the nucleus (Fig. 30), the nucleus of an isotope the neon atom is formed (there
are 0.27% of such ones in Nature). When the twelfth neutron is connected to the
neutron in the upper part of the axis of the nucleus, the screening effect of
the neutron is strengthened (Fig. 30,c). There are 9.22% of the neon atoms with
such nucleus in Nature [120]. As it is clear (Fig. 30), the nucleus of the
isotope of the helium atom is arranged in the apex of the nucleus of the neon
atom.
Neon closes the second period of the periodic table. If
we are on a correct way, the nuclei of the following period of chemical
elements should be repeated in their groups. This demand originates from
periodicity of the properties of chemical determined by D.I. Mendeleyev.
Periodicity of chemical properties of the elements should be provided by the
electrons, which interact with the protons of the repeated structures of the nuclei.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index