<<
Back to Kanarev's Physchemistry Book Index
9.14. Diagram of
Nucleus of Sodium Atom
Sodium is the eleventh element in the
periodic table. It is situated in the first group of this table [2]. It means that the nucleus of this atom should
have the elements of the nucleus of the lithium atom (Fig. 23). In Nature, 100%
of the atoms of this element have the nuclei with eleven protons and twelve neutrons
(Fig. 31). There are isotopes of this element with various periods of half-life
[120], [121].
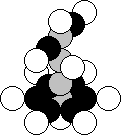
Fig. 31. Diagram of the nucleus of the sodium atom
The upper part of the nucleus of the
sodium atom (Fig. 31) contains the elements of the composition of the nucleus
of the lithium atom (Fig. 23, b), that’s why lithium and sodium are situated in
the same group of the periodic table [2],
[120], [121].
9.15. Diagram of Nucleus of Magnesium Atom
Magnesium is the twelfth element in
the periodic table (Fig. 32). It is situated in the second group of this table,
that’s why the elements of the nucleus of the beryllium atom should be in the
structure of its nucleus (Fig. 24, b). In Nature, 78.99% of the nuclei of the
magnesium atoms contain 12 protons and 12 neutrons (Fig. 32, a) [120].
Let us pay attention to the flat
structure of the nucleus of the beryllium atom (Fig. 24). There are five
neutrons in one plane, and four protons are connected to them. The same
structure is formed in the composition of the nucleus of the magnesium atom (Fig.
32, a). The axial neutrons are shown in grey.
There are twelve protons and twelve
neutrons in the nuclear structure. The twelfth proton is situated in the axis
of the nucleus in its power part. In Nature, 10.00% of the nuclei of the
magnesium atom have the thirteenth neutron (Fig. 32,b), which is connected to
the lower axial neutron. The fourteenth neutron is situated over the upper
axial proton. In Nature, there are 11.01% of the magnesium atoms, whose nucleus
has 14 neutrons [120], [121].
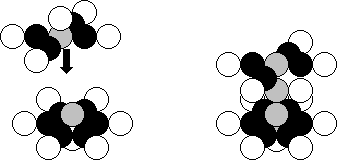
a) b)
Fig. 32. Diagram of the nucleus of the magnesium atom
9.16. Structure of
Nucleus of Aluminium Atom
Aluminium is the thirteenth element of
the periodic table. In Nature, 100% of the atoms of this element have 13 protons
and 14 neutrons. The nuclei with large number of the neutrons belong to
short-life isotopes of this element. As aluminium is included into the third group
of the periodic table, it should contain
the elements of the nucleus of the boron atom. The structure of this
nucleus is given in Fig. 25, a. In Fig. 33, the structure of the nucleus of the
aluminium atom with the specified part,
in which the nuclei of the boron atom are present.
Thus, in the structure of more complicated nuclei the
structures of more simple nuclei are repeated in accordance with the arrangement
of chemical elements according to the groups of the periodic table by D.I.
Mendeleyev [2].
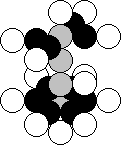
Fig. 33. Diagram of structure of the nucleus of aluminium atom
9.17. Structure of
Nucleus of Silicon Atom
Silicon is the fourteenth element. Its
stable nucleus (there are 92.23% of
such nuclei) contains 14 protons and 14 neutrons (Fig. 34). As silicon is
included in the fourth groupp of the
periodic table together with carbon, the nucleus of the carbon atom should be
in the structure of the nucleus of the silicon atom. It can be represented in
two types: the flat on (Fig. 26, a) and the spatial one (Fig. 26, b).
There are 4.67% of the nuclei of the
silicon atoms with one surplus neutron, and there are 3.10% of the nuclei with
two surplus neutrons. One surplus neutron is situated in lower axial part of
the nucleus between the central neutron and lower proton. The second surplus
neutron is situated on the axis as well.
We lack knowledge in chemistry. If we knew better the
properties of the chemical elements, for the study of which we have no time,
the nuclear structure could be presented more exactly. We make the first steps
on this wonderful and interesting way, and we hope that these people who follow
us will describe more exactly the details, which have remained unclear for us.
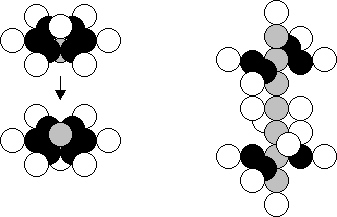
a)
b)
Fig. 34.
Structure of the nucleus of the silicon atom
9.18. Structure of Nucleus of Phosphorus Atom
Phosphorus is the fifteenth element of the periodic table
[2]. It
is situated in fifth group
together with nitrogen atom that is why it contents the nucleus of this atom
(Fig. 27). In Nature, 100% of the nuclei of this chemical
element contain 15 protons and 16 neutrons (Fig. 35). There are short-life
isotopes of this element as well [120], [121]. As it is clear (Fig. 35), the
upper part and the lower one of the nucleus of the phosphorus atom form the
nucleus of the nitrogen atom in the aggregate.
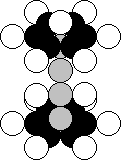
Fig. 35. Structure of the nucleus of the phosphorus atom
9.19. Structure of
Nucleus of Sulphur Atom
Sulphur is the sixteenth element of the periodic table. It is situated in its sixth group together
with oxygen, that’s why the upper part and the lower part of its nucleus form
the nucleus of the oxygen atom in the aggregate (Fig. 28). Each of 95.02% of the nuclei of this element
contains 16 protons and 16 neutrons. In Fig. 36, the structure of the main nucleus
of this element, which has 16 protons and 16 neutrons, is shown [120],
[121].
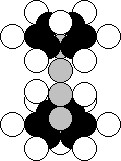
Fig. 36. Structure of the nucleus of the sulphur atom
9.20. Structure of
Nucleus of Chlorine Atom
Chlorine is the seventeenth chemical
element of the periodic table (Fig. 37). Each of 75.77% of the nuclei of this element has 17 protons and 18 neutrons,
and 24.23% of the nuclei have three surplus neutron [120].
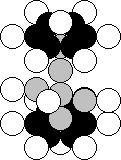
Fig. 37.
Structure of the nucleus of the chlorine atom
3.21. Structure of
Nucleus of Argon Atom
Argon is the eighteenth element in the periodic table. It is included
in the eighth group of this table. Each of 99.60% of the nuclei of this element
contains 18 protons and 22 neutrons. Each of 0.337% of the nuclei has 18 protons
and 18 neutrons, and 0.063% of the nuclei have 18 protons and 20 neutrons
[121].
Let us pay attention to the
structure of the nucleus of the chlorine atom (Fig. 37). It has three tiers.
The upper and the lower tiers consist of the nuclei of the carbon atom. The
middle tier remains unfinished. It is asymmetric. It is necessary to add one
more proton. Then the middle tier will become symmetric. But the electrostatic
forces of repulsion, which exist between the protons of the tiers, will be
increased. In order to reduce the influence of these forces, it is necessary to
increase the distance between the tiers. It is achieved with the help of four
surplus neutrons, and a symmetric nucleus of the argon atom is produced (Fig.
38) [120], [121].
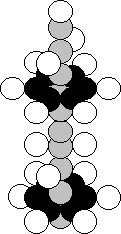
Fig. 38. Structure of the nucleus of the argon atom
9.22. Structure of
Nucleus of Potassium Atom
Potassium (Fig. 39) is the nineteenth element of the periodic
table. The nucleus of this atom contents the nucleus of lithium atom (Fig. 23). In Nature, 93.258% of the nuclei of this
element have 19 protons and 20 neutrons. There are the potassium isotopes with
two and three surplus neutrons.
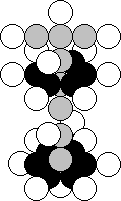
Fig. 39. Structure of the nucleus of the potassium atom
9.23. Structure of Nucleus of Calcium Atom
Calcium is the twentieth element in the periodic table
(Fig. 40). In Nature, 96.94% of the nuclei of the atom of this element have 20
protons and 20 neutrons. The isotopes of this element have 2, 3, 4, 6 and 8
surplus neutrons.
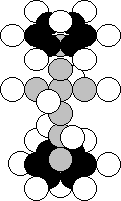
Fig. 40. Structure of the nucleus of the
calcium atom
The analysis of the structure of the nucleus of the
potassium atom shows that it has the same number of the neutrons as the nucleus
of the calcium atom. It means that one free place for the proton should be in
the nucleus of the potassium atom. And we see it. One more tier has appeared in
the nucleus of the potassium atom instead of one middle tier. One of them has
an empty cell for the proton. Let us put a proton in this cells, and we’ll get
a symmetric structure of the nucleus of the calcium atom (Fig. 40) with the
nucleus of the beryllium atom (Fig. 24) [120], [121].
The model looks well, but it should
be taken into consideration that it is built on the grounds of a flat model of
the carbon atom. If we take a spatial model of the nucleus of the carbon atom
as a basis, the structure of the nucleus of the calcium atom can be different.
We leave the possibility of construction of such model to other investigators.
9.24.
Structure of the Nucleus of the Scandium Atom
Scandium is included in the third group of the periodic
table; that’s why the nucleus of the boron atom should be repeated in the structure
of its nucleus. One hundred per cent of
the nuclei of this element contain 21 proton and 24 neutrons (Fig. 41)
[120].
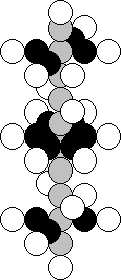
Fig. 41. Diagram of
the scandium nucleus
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index