<<
Back to Kanarev's Physchemistry Book Index
9.4. Diagrams of Nuclei
of Hydrogen Atom
A nucleus of the hydrogen atom is known to
consist of one proton (Fig. 21, a). Hydrogen, which nucleus has one proton and
one neutron, is called deuterium (Fig. 21, b). If the hydrogen atom has one
proton and two neutrons, such atom is called tritium (Fig. 21, c). Let us retrace the process of formation of
the nuclei of deuterium and tritium taking into consideration the
above-mentioned principle of connection of the protons with the neutrons [121].
The approach of the
proton P and the neutron N is shown in Fig. 21, b. The approach takes place due
to the influence of the magnetic forces formed by magnetic fields of unlike
magnetic poles of the proton and the neutron. There are no forces here, which
could prevent the approach of these particles. As a result, a deuterium nucleus
is obtained (Fig. 21, b). If the magnetic fields of the proton and the neutron
are symmetrical, such structure should be stable. There are 0.015% of the
deuterium nuclei in the nature. The approach of the proton and two neutrons and
the formation of the atomic nucleus of tritium are shown in Fig. 21, c. There
are only ![]() of the tritium nuclei
in the nature [27], [120], [130].
of the tritium nuclei
in the nature [27], [120], [130].
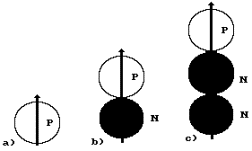
Fig. 21. Diagrams
of approach of the neutrons and the protons due to the influence of their
magnetic forces
If the proton has
the form of torus and the form of the neutron is approximate to a spherical
one, the diagrams of the nuclei of deuterium and tritium can be presented as
the most juxtaposed spherical formations (Fig. 21, b and c) [121].
If we take into
account great strength of magnetic fields of the proton and the neutron near
their geometrical centres, the magnetic forces, which juxtapose these
particles, will correspond to nuclear forces during arrangement of the nuclei
shown in Fig. 21, b and c [121].
Thus, the
insignificant quantity of the nuclei of deuterium and tritium in the nature (as
compared with the number of the nuclei of the hydrogen atoms, which consist of
one proton) shows that the structure of magnetic field of the neutron differs
from the structure of magnetic field of the proton. Let us try to find these
differences using the formation of the atomic nuclei of the chemical elements,
which follow hydrogen, as the examples.
Previously, let us
note an attempt of the author [120] to
use the quantum numbers, which originate from Schroedinger’s equation, for the
formation of the atomic nuclei. The analysis of his attempts shows that their
results have proved to be fruitless as well as the aspiration of the chemists
to distribute the electrons in the atoms according to the levels corresponding
to the marked quantum numbers. Nevertheless, his work concerning the arrangement
of the atomic nuclei of chemical elements is a good reference guide.
9.5.
Diagram of Nuclei of the Helium Atom
Let us pay
attention to a very important difference between the electric fields and the
magnetic fields. It is known that electric fields are easily screened. It is
much more difficult to screen magnetic fields. It appears from this that when
the electric fields of, say, two protons are screened, it is possible to weaken
electrostatic repulsive forces acting between them [121].
What particles screen electrostatic forces of the protons
in the atomic nuclei? The neutrons, of course, nothing, but the neutrons. The
simplest diagram of the helium atom can be such as it is shown in Fig. 22, a.
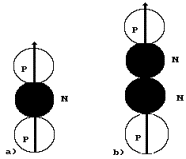
Fig. 22. Diagrams
of the nuclei of the helium atom
If the neutron is
between two protons (Fig. 22, a), it will screen their electrical fields and,
consequently, will weaken electrostatic repulsive forces. As the magnetic
fields are penetrable for the neutron, the presence of the neutron between two
protons will weaken the electrostatic forces, which repel the protons, and
weaken the magnetic forces, which juxtapose them, to a lesser degree. The
structure, which consists of two protons and one neutron and is the nucleus of
the isotope of the helium atom, is formed (Fig. 22, a). There are 0.000138% of
the helium atoms, which have such nucleus, in the nature [120], [121].
The second variant
of formation of the nucleus of the helium atom is shown in Fig. 22, b. Here two
neutrons screen the electric fields of two protons. Such diagram of the nucleus
of the helium atom can be considered more preferable, because in such diagram
of arrangement of the nucleus the electrostatic repulsive forces, which act
between two protons, are weakened greater than in the diagram shown in Fig. 22,
a. Besides, in this diagram both protons have free magnetic poles for the
interaction with the electrons.
It should be noted
that in the majority of the nuclear reactions the nucleus of the helium atom is
released in the form of a positively charged formation called an alpha particle
(Fig. 22, b). Ordinal number 2 of the chemical element helium belongs to a row
of magic numbers, which characterize particular stability of the nucleus of
this element. The next magic numbers are 8 and 20. Later on we’ll consider the
structure of the nucleus of the oxygen atom with the magic number 8 and the
nucleus of the calcium atom with the magic number 20, and we’ll see that their
geometrical symmetry serves a reason of stability of these nuclei [120], [121].
In the variants of
the possible arrangement of the nucleus of the helium atom (Fig. 22), the
neutrons screen a part of electric field lines of the protons. Due to it, the
electrostatic repulsive forces of the protons are reduced. The value of the
magnetic forces, which connect the protons and the neutrons, remains almost the
same, and its provides durability and stability for such assemblage of the
particles.
The number of the
helium atoms, which nuclei consist of two protons and two neutrons (Fig. 22,
b), is 99.999862%. Lifetime of the helium atoms, which nuclei have 4 or 6
neutrons, is calculated in milliseconds [27], [120], [121].
9.6.
Structure of the Nucleus of the Lithium Atom
If the nature is
guided by a principle of geometrical symmetry during the formation of the
atomic nuclei, a question arises: in what sequence does it build a nucleus of
the lithium atom? Of course, the nucleus of simpler helium atom serves as a
base during the construction of the lithium nucleus. In order to make a nucleus
of the lithium atom out of a nucleus of the helium atom, it is necessary to add
one proton and one neutron to the nucleus of the helium atom. If the
arrangement of the nucleus is carried out at the expense of symmetrical
magnetic fields of the proton and the neutron, the diagrams of the nucleus of
the lithium atom will be such as they are shown in Fig. 23, a, b. In the
nature, 92.5% of the nuclei of the lithium atom have three protons and four
neutrons (Fig. 23, a). The rest 7.50% of the nuclei of lithium have three
neutrons and three protons each (Fig. 23, b).
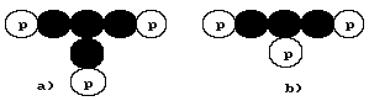
Fig. 23. Diagrams of the nuclei of the lithium atom
Why does Nature prefer
the compositions of the nuclei of the lithium atom, which are shown in Fig. 23, a and b?
Because the protons and the neutrons in the atomic nucleus connect magnetic
force, not nuclear forces. The most important fact is that the majority of
lithium atoms have four neutrons, not three (Fig. 23, a). An unexpected
conclusion results from this diagram: magnetic field of the neutron is formed
by four magnetic poles minimum. This supposition is made, because in the diagram
of Fig. 23, a, the central neutron has three contacts, which correspond to
three magnetic poles. The fourth contact of this neutron is free, it corresponds
to the fourth magnetic pole, to which the neutrons of the isotopes of the
lithium atom are connected [120], [121].
The isotopes of the
lithium atoms can have up to five extra neutrons in the nucleus, but lifetime
of such atoms is calculated in milliseconds. The majority of the lithium atoms
have the nuclei, which are shown in Fig. 23, a. It is explained by the fact
that the protons and the neutrons connect their magnetic forces. Let us pay
attention once again to the quantity of contacts between the neutrons and the protons
in the diagram in Fig. 23, a. Each proton has only one contact with the neutron
being formed by one of its two magnetic poles. One could think that the neutron
has two magnetic poles as well, but the middle neutron, which has three
occupied contacts and one potentially free one, gives us the reason to suppose
that it has a compound magnetic field, which consists of four magnetic poles
minimum.
9.7.
Structure of Nucleus of Beryllium Atom
Let us pay attention to the structure of the
nucleus of the beryllium atom (Fig. 24, a) being built on the supposition that
the nuclear forces connect the protons and the neutrons in the nucleus. It consists of four protons and four neutrons. Its is
rather symmetrical structure. But there are no beryllium atoms with such
nucleus in the nature. The results of the nuclear experimental spectroscopy
show that 100% of the natural beryllium atoms have the nuclei with four protons
and five neutrons (Fig. 24, b). We do not consider the structure of the
artificial isotopes of this element with
a short lifetime [27], [120], [121].
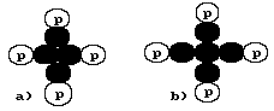
Fig. 24. Diagrams of possible composition
of the nucleus of the beryllium atom
Thus, the absence of the beryllium
nuclei with the nuclear structure, which is shown in Fig. 24, a, in the nature
gives an additional confirmation of the absence of the nuclear forces.
The
structure of the nucleus of the beryllium atom shown in Fig. 24,b gives additional evidences of connection of
the neutrons and the protons by means of unlike magnetic poles of these particles.
This diagram proves significance of the screening functions of the neutron and
complexity of its magnetic field.
In Fig. 24,b the central neutron has four contacts. It means that
the structure of magnetic field of the neutron has four magnetic poles in one
plane: two south poles and two north poles [120], [121].
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index