Published 22.07.2003, updated 29.07.2003
LOW CURRENT PROCESS OF WATER ELECTROLYSIS
Ph. M. Kanarev
E-mail:
kanphil@mail.kuban.ru
Low voltage process
of water electrolysis is known from Faraday’s times. It is widely used in
modern industry. Voltage of 1.6-2.3 volts is operation voltage between the
anode and the cathode of the electrolyzer; current strength is tens and
hundreds of amperes. In accordance with Faraday’s law, energy consumption for
production of one cubic meter of hydrogen is nearly 4 kWh in this case.
The analysis of the water molecule structure (Fig. 1)
worked out by us shows the possibility of water electrolysis at minimal current
and even without it. The protons of the hydrogen atoms in water molecules can
be combined with each other and can form clusters. As a result, an
orthohydrogen molecule is formed (Fig. 2). A question arises: is it possible to
separate this molecule from such cluster? A search of the answer to this
question has lasted nearly three years. Its results are given in Tables 1, 2
and 3.
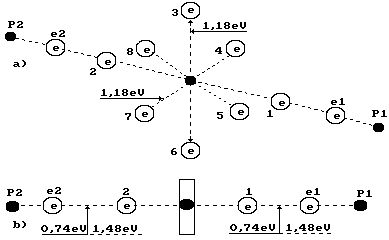
Fig. 1. Water
molecule diagram:
1,2,3,4,5,6,7,8
are numbers of the electrons of the oxygen atom; P1, P2 are the hydrogen atom
nuclei (the protons); e1 and e2 are the electron numbers of the hydrogen atoms
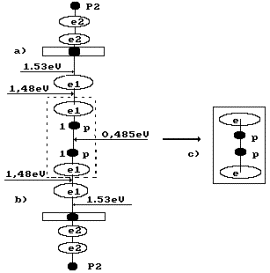
Fig. 2. Formation diagram of orthohydrogen
a) and b) water molecule diagrams; c)
orthohydrogen
It is known that a gram-atom is equal to atomic mass of
substance; a grammolecule is equal to molecular mass of substance. For example,
the grammolecule of hydrogen in the water molecule is equal to two grams; the
gram-atom of the oxygen atom is 16 grams. The grammolecule of water is equal to
18 grams. Hydrogen mass in a water molecule is 2x100/18=11.11%; oxygen mass is
16x100/18=88.89%; this ratio of hydrogen and oxygen is in one liter of water.
It means that 111.11 grams of hydrogen and 888.89 grams of oxygen are in 1000
grams of water.
One liter of hydrogen weighs 0.09 g; one liter of
oxygen weighs 1.47 g. It means that it is possible to produce
111.11/0.09=1234.44 liters of hydrogen and 888.89/1.47=604.69 liters of oxygen
from one liter of water. It appears from this that one gram of water contains
1.23 liters of hydrogen. Energy consumption for production of 1000 liters of
hydrogen is 4 kWh and for one liter 4 Wh. As it is possible to produce 1.234
liters of hydrogen from one gram of water, 1.234x4=4.94 Wh is spent for
hydrogen production from one gram of water now.
Instruments
and equipment used during the experiment
Special experimental low current electrolyzer (Fig.
3); voltmeter of the highest accuracy class (accuracy class of 0.2 GOST
9711-78); ammeter of the highest accuracy class (accuracy class of 0.2 GOST
9711-78)’ electronic scale with scale division value of 0.1 and 0.01 g; stop
watch with scale division value of 0.1 s.

Fig. 3. Low current electrolyzer in the
closed form (in the process of patenting)
The author expresses acknowledgement to
A.I. Tlishev, candidate of technical sciences, for production of this
experimental device and other ones and their testing.
Experimental
results
Table 1
|
Indices |
Sum |
|
1 - duration of the
experiment t, h |
6.000 |
|
2 – readings of voltmeter V,
volts |
3.750 |
|
3 – ammeter readings I,
amperes |
0.020 |
|
4 – power P, watts hour
(P=VxIxτ/60) |
0.450 |
|
5 – continue of experiment
without input energy in 6 series, min |
0.000 |
|
6 – mass difference, grams |
0.52 |
|
7 – mass of evaporated
water, grams |
0.01x6=0.06 |
|
8 – mass of water converted
in hydrogen |
0.46 |
|
9 – specific power P’=P/ |
0.98 |
|
10 – existing specific power P’’, Watt/gram of water |
4.94 |
|
11
– the reducing power on the
production of hydrogen, times K=P’’/P’ |
5.04 |
|
12– quantity of released
hydrogen, ΔМ
=0.46x1.23x0.09=0.051, grams |
0.051 |
|
13 – energy content of
hydrogen being obtained (Е=0.051х142/3,6)=2.008 Wth |
2.008 |
|
14- energy efficacy of low
ampere process of water electrolysis (Eх100/P), % |
446.2 |
Note: In
Table 1, the results of the experiment are given when frequency of nearly 500
Hz has been generated in the power supply
Table 2
|
Indices |
Sum |
|
1 - duration of the
experiment with input energy in 6 series t, min |
6x30=180.0 |
|
2 – readings of voltmeter V,
volts |
3.750 |
|
3 – ammeter readings I,
amperes |
0.022 |
|
4 – power P, watts hour
(P=VxIxτ/60) |
0.247 |
|
5 – continue of experiment without
input energy in 6 series, min |
6x30=180.0 |
|
6 – mass difference, grams |
0.45 |
|
7 – mass of evaporated
water, grams |
0.1x6=0.06 |
|
8 – mass of water converted
in hydrogen |
0.39 |
|
9 – specific power P’=P/ |
0.63 |
|
10 – existing specific power P’’, Watt/gram of water |
4.94 |
|
11
– the reducing power on the
production of hydrogen, times K=P’’/P’ |
8.40 |
|
12– quantity of released
hydrogen, ΔМ
=0.39x1.23x0.09=0.043, grams |
0.043 |
|
13 – energy content of hydrogen
being obtained (Е=0.043х142/3,6)=1.70 Wth |
1.70 |
|
14- energy efficacy of low
ampere process of water electrolysis (Eх100/P), % |
689.0 |
Note: In
Table 2, the results of the experiment are given when no additional frequency has
been generated by the power supply
Table 3
|
Indices |
Sum |
|
1 - duration of the
experiment with input energy in 6 series t, min |
6x5=30 |
|
2 – readings of voltmeter V,
volts |
13.60 |
|
3 – ammeter readings I,
amperes |
0.020 |
|
4 – power P, watts hour
(P=VxIxτ/60) |
0.136 |
|
5 – continue of experiment
without input energy in 6 series, min |
6x55=330 |
|
6 – mass difference, grams |
0.44 |
|
7 – mass of evaporated
water, grams |
0.01x6=0.06 |
|
8 – mass of water converted
in hydrogen |
0.38 |
|
9 – specific power P’=P/ |
0.358 |
|
10 – existing specific power P’’, Watt/gram of water |
4.94 |
|
11
– the reducing power on the
production of hydrogen, times K=P’’/P’ |
13.80 |
|
12– quantity of released hydrogen,
ΔМ
=0.38x1.23x0.09=0.042, grams |
0.042 |
|
13 – energy content of
hydrogen being obtained (Е=0.042х142/3,6)=1.66 Wth |
1.66 |
|
14- energy efficacy of low
ampere process of water electrolysis (Eх100/P), % |
1220.0 |
Note: In Table
3, the results of the experiment are given when frequency of nearly 500 Hz has
been generated in the power supply
First of
all, we should note that the anode and the cathode are made of one and the same
material: steel. It excludes the possibility of formation of a galvanic cell.
If we analyze Tables 1, 2 and 3, we’ll see the electrolysis process takes place
at very low current of 0.02 A; that’s why it has been called low current one.
Further, this process consisted of two cycles in some experiments; in one
cycle, the electrolyzer is connected to the power line; in another cycle, it is
disconnected.
Gas
generation process is manifested by release of the bubbles being formed. The
bubbles go on being released after the electrolyzer is disconnected from the
supply line (Tables 2 and 3). When the electrolyzer is de-energized, gas
release intensity is reduced, but it is not stopped during many hours. It is
proved by the fact that electrolysis takes place at the expense of potential
difference on the electrodes. It should be noted that small potential
difference takes place on the electrodes of the empty electrolyzer and
immediately after it has been charged with electrolyte prior to its connection
to the supply line.
If water
electrolysis took place only at the expense of the electrons emitted by the
cathode, i.e. according to Faraday’s law, current value would be greater and
the stock of these electrons would give out after the electrolyzer was
de-energized and the gas release process would be stopped.
After
electrolyzer de-energizing, gas release during a long period of time proves the
fact that the molecules of oxygen and hydrogen are formed without the electrons
emitted by the cathode, i.e. at the expense of the electrons of the water
molecule itself. Let us analyze this process
in detail.
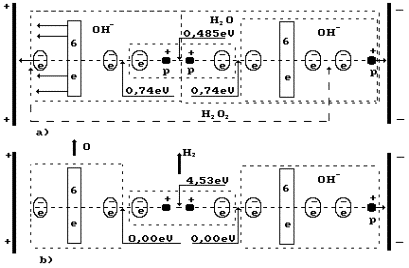
Fig. 4. Diagram of low current electrolysis
process
In Fig. 4,
the anode is shown leftward and the cathode is shown rightward. The proton of the
hydrogen atom in water molecule is oriented to the cathode; another proton of
this molecule is connected with the proton of the ion OH` (leftward). As a result, a cluster chain is formed; rightward, the
water molecule H2O is situated; leftward, the ion OH` is situated (Fig. 4, a); the orthohydrogen
molecule H2 is in the center (Fig. 4, a, b). Both protons of the
hydrogen molecule are connected by energy 0.485 eV corresponding to water
molecule cluster formation energy at the temperature of 20°C. Binding
energies of the left electron of the hydrogen molecule with the electron of the
oxygen atom and the right electron with the electron of the ion OH` are equal to 0.74 eV (Fig. 1).
Thus, the
complex cluster chains with strict orientation between the anode and the
cathode are formed in the electrolytic solution under the influence of the
electrostatic field.
Let us pay
attention to the fact that the axis electron of the oxygen atom and its six ring electrons of the ion OH` are attracted to the anode simultaneously
(Fig. 4, a). Electrostatic forces attracting six ring electrons to the anode
deform the electrostatic field in such a way that the axis electron comes to
the nucleus of the oxygen atom, and six ring electrons withdraw from the atomic
nucleus. As the electron withdrawal process from the atomic nucleus is an
endothermic one, six ring electrons absorb 1.18 eV x 6 = 7.08 eV (s. Fig. 5).
It will automatically transform both axis electrons of the oxygen atom to the
energy levels corresponding the excited state of the oxygen atom. Energy
absorbed by the ring electrons of two atoms of oxygen is 7.08 x 2 = 14.16 eV.
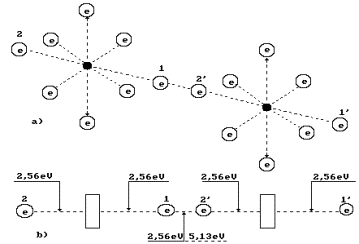
Fig. 5. Diagram of binding energy
distribution between the electrons in the oxygen molecule
When two
oxygen atoms have been separated from two cluster chains, their two axis
electrons form a covalent bond releasing 5.13 eV of energy (s. Fig. 5). Other
two electrons of the oxygen atoms arranged at the ends of the axis of the
molecule O2 will go to the energy levels with binding energies of
2.56 eV having emitted (2.56-0.74) x 2 = 6.92 eV.
Hydrogen
molecule fusion energy is (4.53-0.485-1.48x2)=1.085 eV taking into
consideration a binding energy change between its protons and electrons (Fig.
4) and conversion of orthohydrogen into parahydrogen. Fusion energy of two
molecules of parahydrogen is 1.085 x 2 = 2.170 eV. As a result, total energy of
fusion of one oxygen molecule and two hydrogen molecule is
(5.13+6.92+2.170)=14.22 eV.
Difference
between absorbed energy and emitted energy will be (14.22-14.16)=0.06 eV. This
is exothermic energy. If we take into account that nearly 0.5 liter of hydrogen
has been released during the experiment, thermal energy should be generated.
![]() (1)
(1)
Solution
mass in the electrolyzer was nearly 0.3 kg. If we take it into consideration,
the solution should be heated by
![]() (2)
(2)
The experiment
has shown that when the electrolyzer is energized, solution temperature is
increased by 1.5-2°C. When the electrolyzer is de-energized and the
electrolysis process goes on, solution temperature is reduced. When the
electrolyzer is de-energized, potential on the electrodes is reduced gradually.
It means that the initial stock of the electrons on the cathode is reduced as
well. It takes place due to restoration of the ions of an alkaline metal. When
the ions are restored, they take electrons from the cathode and reduce
potential between the electrodes. It is manifested by cathode surface color. It
acquires color of the alkaline metal being used.
Thus, low
ampere electrolyzer can operate in two modes: connected to the supply line and
disconnected from it. When the electrolyzer is connected to the supply line, a
part of gases is released using the electrons of the cathode, and a part is
released without the use of these electrons. When the electrolyzer is
disconnected from the supply line, the cathode electrons are spent for
restoration of alkaline metals.
In Fig. 4,
a, the boundaries of the hydrogen peroxide molecule H2O2
are shown in the cluster chain (Fig. 6). It is clear (Fig, 4) that the hydrogen
peroxide molecule is released only when a hydrogen atom being in contact with
the cathode is released from the cluster chain. It takes place when voltage is
increased. Hydrogen peroxide is released and interacts with the anode material;
if it is iron, foam with red flakes is formed at once. Foam is an apparent
feature of spontaneous gas release process disturbance.
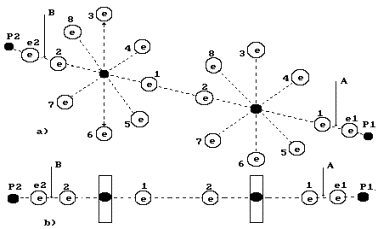
Fig. 6. Diagram of the model of hydrogen
peroxide H2O2
Involuntarily,
the results being obtained form an aspiration to find an analogy of the
described low ampere process of water electrolysis in Nature.
It is
known that carbon dioxide CO2 is absorbed during photosynthesis. It
is considered that carbon C of the molecule CO2 is used for plant cell construction, and oxygen O2
is released. Now we have every reason to doubt at it and to suppose that the
molecule CO2 is used totally
for plant cell construction. Water molecules release oxygen; the hydrogen atoms
of water molecules are used as connecting links of the molecules, from which
the plant cells are constructed. This process is similar to the process shown
in Fig. 4.
CONCLUSION
Simplicity
and 100% reproducibility of the experiments being described afford ground for
the fact that mankind has got a chance to avoid energy famine and environmental
crisis.
REFERENCES
1. Ph.M. Kanarev.
The Foundation of Physchemistry of Microworld.
Krasnodar,
2002. 320 pages.
2. Ph.M. Kanarev. The Foundation of Physchemistry of Microworld.
The second edition (in Russian) hppt://www.ikar.udm.ru/sb28-2.htm
3. Ph.M. Kanarev. The Foundation of Physchemistry of Microworld.
The second edition (in English). http://book.physchemistry.innoplaza.net
4. Ph.M. Kanarev. Energy Balance of Fusion Processes of Molecules of
Oxygen, Hydrogen and Water. http://www.n-t.org/tp/ts/eb.htm
5. Ph.M. Kanarev. Energy Balance of Fusion Processes of Molecules of
Oxygen, Hydrogen and Water. http://Kanarev.innoplaza.net
Webmaster: j_hartikka@hotmail.com
Low Current Process of Water Electrolysis by Prof. Kanarev:
http://Kanarev.lowcurrent.innoplaza.net
http://hydrogen.innoplaza.net
<< Kanarev´s Page