Published 19.12.2003
Updated 04.11.2005
with drawing of the cell.
LOW CURRENT ELECTROLYSIS OF
WATER
Ph. M. Kanarev
E-mail: kanphil@mail.ru
An
interest to hydrogen energetic is being increased of late years. It is
explained by the fact that hydrogen is an inexhaustible and
environmental-friendly energy carrier. But the implementation of these
properties is slowed down by large energy consumption for its production from
water. The most modern Electrolyzers
consume 4.0 kWh per cubic meter of this gas. Electrolysis process takes place
by voltage of 1.6-2.0 V and current strength of dozens and hundreds of amperes.
When one cubic meter of hydrogen is burnt, 3.55 kWh of energy is released [1].
Many
laboratories in the world are busy solving a problem of a reduction of energy
consumption for hydrogen production from water, but there are no significant
results. In the meantime, a money-saving process of decomposition of water
molecules into hydrogen and oxygen exists in the nature. This process takes
place during photosynthesis. Hydrogen atoms are separated from water molecules
and are used as connecting links while forming organic molecules, and oxygen is
released into the air.
A
question emerges: is it possible to model an electrolytical process of water
decomposition into hydrogen and oxygen, which takes place during
photosynthesis? A search of a reply to this question has resulted in a simple
structure of a cell (Fig. 1), in which the process takes place by voltage of
1.5-2.0 V between the anode and the cathode and amperage of 0.02 amperes [1],
[2].
|
|
Fig. 1. Model of a low current cell of the electrolyzer (at the stage of patenting) |
The electrodes of the cell are made of steel. It helps to avoid the
phenomena, which are appropriate to a galvanic cell. Nevertheless, at the cell
electrodes a potential difference of nearly 0.1 V takes place in complete
default of electrolytic solution in it. When the solution is charged, the
potential difference is increased. The positive sign of the charge appears on
the upper electrode always, and the negative sign appears on the lower one. If
a direct current source generates pulses, gas output is increased.
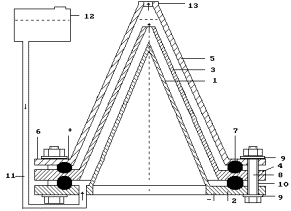
Fig. 1a.
Low current electrolyzer (Patent #
2227817)
As
a laboratory model of the low current electrolyzer cell generates small
quantity of gases, a solution mass change definition method during the
experiment and further calculation of released hydrogen and oxygen is the most
reliable method of definition of their quantity.
It is known that a gram atom is equal to atomic mass of substance; a gram molecule is equal to molecular mass of substance. For example, the gram molecule of hydrogen in the water molecule is equal to two grams; the gram-atom of the oxygen atom is 16 grams. The gram molecule of water is equal to 18 grams. Hydrogen mass in a water molecule is 2x100/18=11.11%; oxygen mass is 16x100/18=88.89%; this ratio of hydrogen and oxygen is in one liter of water. It means that 111.11 grams of hydrogen and 888.89 grams of oxygen are in 1000 grams of water.
One liter of hydrogen weighs 0.09 g; one liter of oxygen weighs 1.47 g. It means that it is possible to produce 111.11/0.09=1234.44 liters of hydrogen and 888.89/1.47=604.69 liters of oxygen from one liter of water. It appears from this that one gram of water contains 1.23 liters of hydrogen. Energy consumption for production of 1000 liters of hydrogen is 4 kWh and for one liter 4 Wh. As it is possible to produce 1.234 liters of hydrogen from one gram of water, 1.234x4=4.94 Wh is spent for hydrogen production from one gram of water now.
Instruments and equipment
used during the experiment
Special experimental low current electrolyzer (Fig. 3); voltmeter of the highest accuracy class (accuracy class of 0.2 GOST 9711-78); ammeter of the highest accuracy class (accuracy class of 0.2 GOST 9711-78) electronic scale with scale division value of 0.1 and 0.01 g; stop watch with scale division value of 0.1 s.
Table 1
Experimental results
|
Indices |
Amount |
|
1 period of service of the
electrolyzer connected to the line,
in six cycles t, min |
6x10=60.0 |
|
2 voltmeter readings V, volts; |
11.00 |
|
2 oscillograph readings V, volts; |
0.062 |
|
3 ammeter readings I, ampere; |
0.020 |
|
3 oscillograph readings, I, ampere; |
0.01978 |
|
4 energy
consumption according to the voltmeter and ammeter (P=VxIxτ/60),
Wh; |
0.220 |
|
4 energy consumption according to
oscillograph readings (P=VxIx τ/60) Wh; |
0.00124 |
|
5 period of service of the electrolyzer
disconnected from the line, in six cycles, min |
6x50=300.0 |
|
6 solution mass change m, grams |
0.60 |
|
7
evaporating water mass m, grams |
0.06 |
|
8 mass of water passed into gases,
m=m-m, grams |
0.54 |
|
9 energy consumption per gram of water passed
into gases according to the readings of the voltmeter and ammeter E=P/m,
Wh/gram of water |
0.407 |
|
9
energy consumption per gram of water passed into gases according to
oscillograph readings E=P/m, Wh/gram of water |
0.0023 |
|
10 existing energy consumption per gram of
water passing into gases E, Wh/gram of water |
4.94 |
|
11 reduction of
energy consumption for hydrogen production from water according to the
readings of voltmeter and ammeter
K=E/P, fold |
12.14 |
|
11 reduction of
energy consumption for hydrogen production from water according to the
oscillograph readings K=E/P, fold |
2147.8 |
|
12- released hydrogen quantity ΔМ=0.54x1.23x0.09=0.06,
gram |
0.06 |
|
13
energy content of produced hydrogen (W=0.06х142/3.6) =2.36, Wh |
2.36 |
|
14 energy
effectiveness of water electrolysis process according to the readings of the
voltmeter and the ammeter (Wх100/P), % |
1072.7 |
|
14 - energy
effectiveness of water electrolysis process according to the oscillograph
readings (Wх100/P), % |
190322.6 |
Oscillogram samples
|
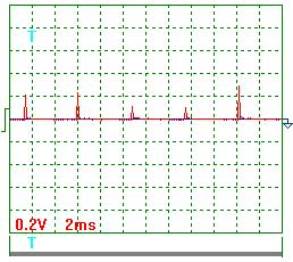
Fig. 2. Voltage |
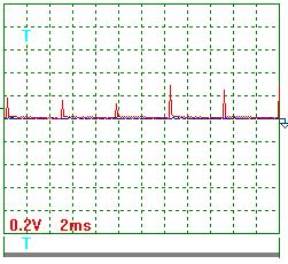
Fig. 3. Voltage |
|
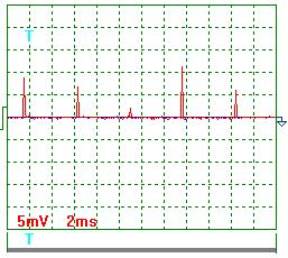
Fig. 4. Current |
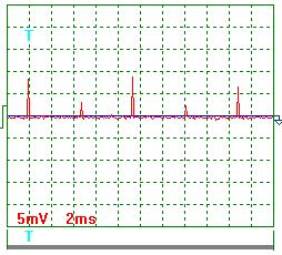
Fig. 5. Current |
Voltage oscillogram processing
results (Figs 2 and 3). Taking into consideration the scale factor, which is equal to 10, we'll
find a mean value of voltage pulse amplitude
![]() =[(0.20+0.24+0.12+0.10+0.30+0.18+0.16+0.12+0.30+
0.24+0.30)/11] x10=2,05 V .
=[(0.20+0.24+0.12+0.10+0.30+0.18+0.16+0.12+0.30+
0.24+0.30)/11] x10=2,05 V .
Pulse period Т=(24х2)/10=4.8 ms.
Pulse duration ![]() =(2х1.45)/10=0.29 ms.
=(2х1.45)/10=0.29 ms.
Pulse frequency ![]() =(1/0.001x4.8)=208.3 Hz.
=(1/0.001x4.8)=208.3 Hz.
Pulse period-to-pulse duration ratio ![]() =48/0.29=16.55.
=48/0.29=16.55.
Duty factor ![]() =0.5/16.55=0.0302.
=0.5/16.55=0.0302.
Equivalent mean component of voltage pulses
calculated according to the oscillograph readings ![]() =2.05х0.0302=0.062 V. At that time, the voltmeter
readings were 11.0 V.
=2.05х0.0302=0.062 V. At that time, the voltmeter
readings were 11.0 V.
Current oscillogram
processing results (Figs 4 and 5). Taking
into consideration the scale factor, which is equal to 10, and resistance of
0.1 Ohm resistor we'll find a mean value of current pulse amplitude
![]() ={[(9.0+7.0+2.0+11.5
+6.0+8.5+3.5+9.0+2.5+6.5)/10]x10}/0.1=655мА =0.655 А.
={[(9.0+7.0+2.0+11.5
+6.0+8.5+3.5+9.0+2.5+6.5)/10]x10}/0.1=655мА =0.655 А.
Mean current in the electrolyzer supply circuit is ![]() =0.655х0.0302=0.01978А =0.02А. The ammeter readings are 0.02 А.
=0.655х0.0302=0.01978А =0.02А. The ammeter readings are 0.02 А.
A
question emerges at once: why is current value according to the readings of the
ammeter and oscillograph the same and voltage value according to the oscillograph readings is 177.4fold less
than according to the voltmeter readings? A series of additional experiments
accompanying this question is shown that a low current electrolyzer cell is a
capacitor being discharged gradually under the influence of electrolytical
processes, which take place in it. A value of this discharge is compensated by
the pulses of voltage, which mean value is considerably less than a constant
value of charge voltage of this capacitor.
Thus,
the voltmeter shows a capacitor charge voltage value, and the oscillograph shows a value of its recharge,
which characterizes the energy consumed by the cell from the line. It appears
from this that in order to calculate energy consumed by the low current
electrolyzer cell from the line it is necessary to use voltage, which is
registered not by the voltmeter, but by the oscillograph. As a result, energy
consumption for hydrogen production from water in case of low current
electrolysis are reduced not 12fold, but almost 2000fold.
Thus,
a small value of current 0.02 A and voltage 0.062 V allows us to suppose that
in the low current electrolyzer the water electrolysis process is similar to
the process, which takes place during photosynthesis. At photosynthesis,
hydrogen separated from the water molecule is used as a connecting link while
organic molecule formation, and oxygen is released in the air. At low current
electrolysis, both hydrogen and oxygen are released in the air.
Fruitfulness of this attractive hypothesis should be checked not once, but now it is the only one, which gives a satisfactory explanation of an unusual experimental result.
Note: gas release is clearly seen during several
hours after the cell is disconnected from the line.
Conclusion
Energy efficiency index of the low current electrolysis should be
refined, but in any case it will be greater than 10, thats why there is every
reason to think that a way to production of inexpensive hydrogen from water and
transition to hydrogen energetic is opened.
REFERENCES
1. Kanarev
Ph.M. The Foundation of Physchemistry
of Microworld. The third edition.
Krasnodar: KSAU, 2003. http://Kanarev.innoplaza.net (In
Russian, Part 1, Part 2).
2. Kanarev
Ph.M. The Foundation of Physchemistry
of Microworld. The second edition. (In
English). http://book.physchemistry.innoplaza.net
Webmaster: j_hartikka@hotmail.com
Low Current Electrolysis of Water by Prof. Kanarev
<< Kanarev΄s Page