<<
Back to Kanarev's Physchemistry Book Index
12. PLASMA –
ELECTROLYSIS OF WATER
12.1.
Plasma - Electrolytic Process
Electrolytic
processes are known for a long time, and they are widely used in chemical
industry. Plasma - electrolytic processes have been found quite recently,
that’s why there is neither physical, no chemical theory of these processes.
The preliminary analysis shows that a complete description of plasma -
electrolytic process can be based neither on purely physical notions, nor
purely chemical ones. These are interconnected physical and chemical processes,
that’s why it is possible to subdivide them into physical processes and
chemical ones only conditionally.
A plasma - electrolytic
reactor is a device, which is made of dielectric material (Figures 85-88). The working
solution is fed into the space between the electrodes. If voltage is increased
it results in the change of strength of current in a chain, which
characteristic appropriateness is shown in Fig. 84.
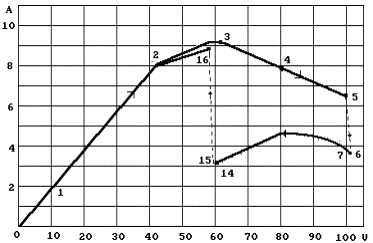
Fig. 84. Volt - ampere
characteristic, which corresponds to Table 38
Table 38. Test results of
reactor No. 1 when 8.74 litres per hour of 1-normal solution HCl is consumed. its temperature at the input
being 23.0°C
|
Rated points |
Voltage, V |
Current, A |
Input Energy, kJ |
Temperature of the solution,
C |
Output Energy, kJ |
Index efficiency,% |
|
1 |
2 |
3 |
4 |
5 |
7 |
9 |
|
1 |
10 |
1.7 |
61.2 |
24 |
36.6 |
59.8 |
|
2 |
40 |
8.2 |
1180.8 |
49 |
952.1 |
80.6 |
|
3 |
58.5 |
9.15 |
1927.0 |
73 |
1831.0 |
95.0 |
|
4 |
80 |
7.85 |
2260.8 |
82 |
2160.6 |
95.6 |
|
5 |
100 |
6.65 |
2394.0 |
83.5 |
2215.5 |
93.0 |
|
6 |
102 |
3.75 |
1377.0 |
81 |
2121.1 |
154.0 |
|
7 |
85 |
4.7 |
1438.2 |
69 |
1684.5 |
117.1 |
|
8 |
76 |
4.3 |
1176.5 |
65 |
1538.0 |
130.7 |
|
9 |
68.5 |
3.75 |
924.7 |
55 |
1171.8 |
126.7 |
|
10 |
88 |
4.5 |
1425.6 |
71 |
1757.8 |
123.3 |
|
11 |
92 |
4.2 |
1391.0 |
71 |
1757.8 |
126.4 |
|
12 |
94 |
4.4 |
1489.0 |
71.5 |
1776.1 |
119.3 |
|
13 |
98 |
4.2 |
1481.8 |
71 |
1757.8 |
118.6 |
|
14 |
68 |
3.9 |
954.7 |
56 |
1208.5 |
126.6 |
|
15 |
64 |
3.3 |
760.3 |
50 |
988.7 |
130.0 |
|
16 |
61 |
3.05 |
669.8 |
46 |
842.3 |
126.0 |
|
17 |
57.5 |
9.3 |
1925.1 |
72 |
1794.4 |
93.2 |
Strength of current
is increased when voltage is increased linearly according to Ohm’s law. If
voltage exceeds 40 V, Ohm’s law is violated; if voltage is about 100 V (points
5-6), strength of current is increased spasmodically, and a bright glow
(plasma) appears near the cathode. Further compulsory reduction of voltage
(points 6-15) changes strength of current insignificantly. When voltage is
about 60 V (points 14-15), the cathode glow disappears, strength of current is
increased spasmodically almost up to the former value [71], [81], [109], [189].
Note: energies of
released hydrogen and oxygen as well as emitted light have not been taken into
account.
As hydrochloric
acid has taken part in the process, we’ll need binding energies of valence
electron of chlorine atom during the analysis. Ionization potential of the
first electron of chlorine atom is ![]() = 12.967 eV, and its
binding energy with the atom corresponding to the first energy level is
= 12.967 eV, and its
binding energy with the atom corresponding to the first energy level is ![]() = 15.548 eV
= 15.548 eV
Table 39. Spectrum of the 1th
electron of chlorine atom and its binding energy ![]() with the nucleus
with the nucleus
|
Quantum number |
n |
2 |
3 |
4 |
5 |
6 |
|
|
eV |
9.08 |
11.25 |
12.02 |
12.34 |
12.53 |
|
|
eV |
9.08 |
11.24 |
11.99 |
12.34 |
12.54 |
|
|
eV |
3.28 |
1.46 |
0.82 |
0.52 |
0.36 |
12.1.1.
Physical Model of
Plasma - Electrolytic Process
In order to find
out the physical model of the process, it is desirable to observe how it takes
place. For this purpose a special reactor was manufactured, which cathode
chamber was made in the form of a hole in flat acrylic plastic with the
thickness of 24 mm. The needle cathode made of tungsten was introduced in the
hole from above, and working solution was fed from beneath and arrived from a
side hole. Transparency of acrylic plastic allows to watch some details of the
plasma - electrolytic process in various modes of the reactor operation. Prior
to describe the observation results, let us characterize the main
«participants» of the plasma - electrolytic process: the electrons, the protons,
the atoms, the ions and water molecules.
Earlier we have
shown that the electron radius is its main geometrical parameter. It is equal
to Compton wave-length ![]()
![]() (172), and it is
changed insignificantly during energy transitions of the electron in the atom.
We have found out that the proton radius is equal to
(172), and it is
changed insignificantly during energy transitions of the electron in the atom.
We have found out that the proton radius is equal to ![]() (230). It is by three orders of magnitude less that the electron radius
[109].
(230). It is by three orders of magnitude less that the electron radius
[109].
Hydrogen atom is
the next «participant» of the process. Its size is variable. It is increased
with the temperature increase of the environment, which surrounds the atom.
When the electron in the atom is on the first energy level, the distance
between the proton (atomic nucleus) and the electron is equal to ![]() (240), i.e. it is equal
approximately to one Angstrom. The size of hydrogen atom in non-exited state is
by five orders of magnitude greater than the proton and by two orders of magnitude
greater than the size of the electron.
When the electron goes to the third energy level, the distance between
it and the proton in hydrogen atom is increased and becomes equal to 9.54×10-10 (244).
(240), i.e. it is equal
approximately to one Angstrom. The size of hydrogen atom in non-exited state is
by five orders of magnitude greater than the proton and by two orders of magnitude
greater than the size of the electron.
When the electron goes to the third energy level, the distance between
it and the proton in hydrogen atom is increased and becomes equal to 9.54×10-10 (244).
We have founds out
that the sizes of all atoms in non-exited state are near to the size of
hydrogen atom. Consequently, the size of oxygen is near to one Angstrom. The
sizes of the ions (we do not consider the proton as the ion) and the molecules
are several times greater than the sizes of the atoms and depend of the numbers
of energy levels, on which valence electrons uniting the atoms into the
molecules are situated.
Let us consider the
structure of water molecules (Figs 72-74) and binding energies of the electrons with the
nuclei of the atoms of hydrogen, oxygen
and chlorine (Table 35, 36, 39). It means that a separation of the hydrogen atom and one proton from water
molecule and from the molecule of hydrochloric acid (HCl) are the phenomena of
equal probability, because they have similar binding energies on energy levels
of the same name.
If we analyse the
data of Fig. 84
and Table 38,
we can form the following physical model of plasma - electrolytic process. When
voltage is increased up to 60 V, a well known ion conductivity operates in the
solution. At such potential water molecules contacting with the cathode by
positively charged protons of hydrogen atoms dissociate into molecular hydrogen
![]() and hydroxyl ions
and hydroxyl ions ![]() (Fig. 75). In this case a usual process of water
electrolysis takes place [109].
(Fig. 75). In this case a usual process of water
electrolysis takes place [109].
When voltage is
increased, hydrogen atoms and their protons begin to be separated from water
molecules. At first separate streamers (sparks) appear in the solution near the
cathode. It points out to the fact that the protons of hydrogen atoms are
separated from water molecules and during their movement to the cathode are
united again with the electrons producing new atoms of hydrogen. Further
increase of voltage increases quantity of the protons, which have been separated
from water molecules, and plasma of hydrogen
atoms is formed near the cathode (points 5, 6). The electrons of hydrogen atoms
are in an exited state at this moment and move from high energy levels to low
energy levels generating the light of Balmier spectral lines. One can judge by
intensity of these lines, between which energy levels of hydrogen atoms the
largest quantity of electron moves.
Visual analysis of
the whole spectrogram (only a part in shown in Fig. 81) demonstrates that the
largest quantity of electrons in hydrogen atoms moves from the third to the second
energy level (the light bright band to the left of Fig. 82). The light zone
near this band to the right confirms the simultaneous formation of hydrogen
molecules [60], [61], [62].
As voltage is
reduced (points 7-14) the volume of plasma is reduced, energy levels of the
electrons of hydrogen atoms, on which they stop, move off from the protons, energy of emitted photons
is reduced, wave-length is increased, and colour of plasma is charged from
bright white to red. Then the moment takes place (point 15) when the potential
on the electrons is not enough for the separation of the protons from water
molecules, and the process goes out slowly returning the system into the
initial state of ion conductivity (Fig. 84).
If we analyse Table
38 and Fig. 84,
we see that the data on the mode corresponding to point 6 are of the greatest
interest. This mode has been formed spontaneously. Stable plasma is absent in
point 5, only glimmer can be observed near the cathode. Then in a certain
period of time current is reduced spontaneously, and stable plasma appears at
once.
Plasma being formed
limits the contact of the solution with the surface of the cathode (it
increases resistance in cathode - solution circuit). As a result, the value of
current is reduced sharply and remains such till energy of plasma and applied
voltage is enough for the separation of the protons from water molecules.
Hydrogen atoms
unite into molecules on «plasma - solution» boundary. Their further fate
depends on availability of oxygen atoms. If they exist, water molecules are
formed with the characteristic micro - explosions, which generate noise in some
modes of the reactor operation. If there are no oxygen atoms near the cathode
or they have been united into oxygen molecules, hydrogen molecules are mixed
with oxygen molecules and form the so-called «blasting mixture», which is
removed from the cathode together with water vapours.
If voltage is
increased after appearance of plasma (Fig. 84, point 6), plasma temperature
is increased, and the spike of tungsten cathode becomes bright white at first,
then it starts burning. It is easy to observe this process through transparent
acrylic plastic of the reactor. The larger voltage, the more intensive is
burning (melting) of the cathode. It is known that melting point of tungsten
is 3382°C, and its boiling
point is 6000°C.
Thus, atomic
hydrogen is a source of plasma in plasma - electrolytic process. Alternating
electric field keeps hydrogen atom in an exited state forming its plasma with
the temperature of (5000...10000)°C. Intensity of
this plasma will depend on applied voltage and on the consumption of the
solution, which flows about the cathode. The greater applied voltage and the
greater the consumption of the solution, the more intensive plasma is.
12.1.2.
Chemical Model of
Plasma - Electrolytic Process
Starting to
consider a chemical model of the plasma - electrolytic process we have to point
out that modern chemistry does not know an abundance of energy levels of each
electron and an abundance of binding energies between the atoms in the
molecules. We do not know how the values of binding energies of hydrogen atoms
with oxygen atoms in water molecule have been obtained before our
investigations (with the help of the calculations or experiments), but we have
shown that these energies do not correspond the energies of dissociation of
water molecules during its low voltage electrolysis, i.e. they do not
correspond to energy consumption during water decomposition into hydrogen and
oxygen. We are faced with the problem: what to do next? Shall we trust these
and other calculation results of modern chemistry or shall we cast doubt on
them?
As atomic hydrogen
exists at the temperature of (5000...10000)°C [52], plasma with such
temperature is formed in the cathode zone. Plasma will exist only under the
condition of sufficient density of hydrogen atoms in the given volume. In order
to fulfil this condition it is necessary to increase density of current on the
cathode. After the formation of hydrogen atoms or their separation from water
molecules they would remain in non-exited state if there were no external
influence. But during the operation of the plasma - electrolytic reactor
hydrogen atoms are at continuous influence of alternating electric field, which
makes hydrogen atoms be in exited sate, it is proved by availability of a
complete set of Balmier spectral lines on a spectrogram. Unfortunately, we have
no complete spectrum of hydrogen atom and know nothing about availability of
Limon spectral lines, Pashen spectral lines, etc., it makes the analysis of the
phenomenon being studied difficult [109].
The
following chemical reactions will take place in the “plasma – solution”
interphase boundary at the same time
![]() . (292)
. (292)
and
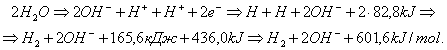 (293)
(293)
If a
molecule of oxygen ![]() is formed near
the anode, energy is released
is formed near
the anode, energy is released
![]() (294)
(294)
In the
model of the reactor, which trial results are given in Table 40, hydrogen and
oxygen escape via the same branch pipe, that’s why endotermic reactions are
possible in it [2]
1- formation of hydrogen peroxide ![]()
![]() (295)
(295)
2 - formation of ozone ![]()
![]() (296)
(296)
3- formation of the ion of
hydroxonium ![]()
![]() (297)
(297)
Unfortunately, we
do not know intensity of both exothermic (292, 293, 294) and endothermic (295, 296, 297) reactions. Solution
temperature change regularity (Table 38) points out to the fact that intensity of endothermic
reactions in the zone of existence of molecular hydrogen (points 3, 4, 5) is
lower than in points 7-15 where plasma of atomic hydrogen is preserved, and
solution temperature is reduced. The reduction of water temperature during
voltage decrease in the experiment (Table 38, points 6-15) is explained by
intensive absorption of heat during the formation of hydrogen peroxide ![]() , ozone
, ozone ![]() and ion
and ion ![]() .
.
The Japanese
investigators Ohmori and Mizuno found dissemination of nickel, chromium and
carbon on the cathode of the plasma - electrolytic reactor [51]. To their mind,
cold nuclear fusion is a source of these chemical elements. Later on we’ll
analyze this phenomenon in detail.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index